The IDWP aims to promote and conduct educational activities and clinical investigations on all the topics concerning the diagnosis, the prophylaxis and the management of infectious complications following HSCT.
The mission of the IDWP is to share the experience and develop cooperative studies to increase education in the field of diagnosis, prophylaxis and treatment of infectious complications in HSCT patients.
IDWP Members continue their scientific and educational activity in the fields of: bacterial infections, viral infections, fungal infections including pneumocystis jiroveci infections, parasitic infections, vaccinations, protective environment and guidelines from the area of transplant infectious diseases.
IDWP Team







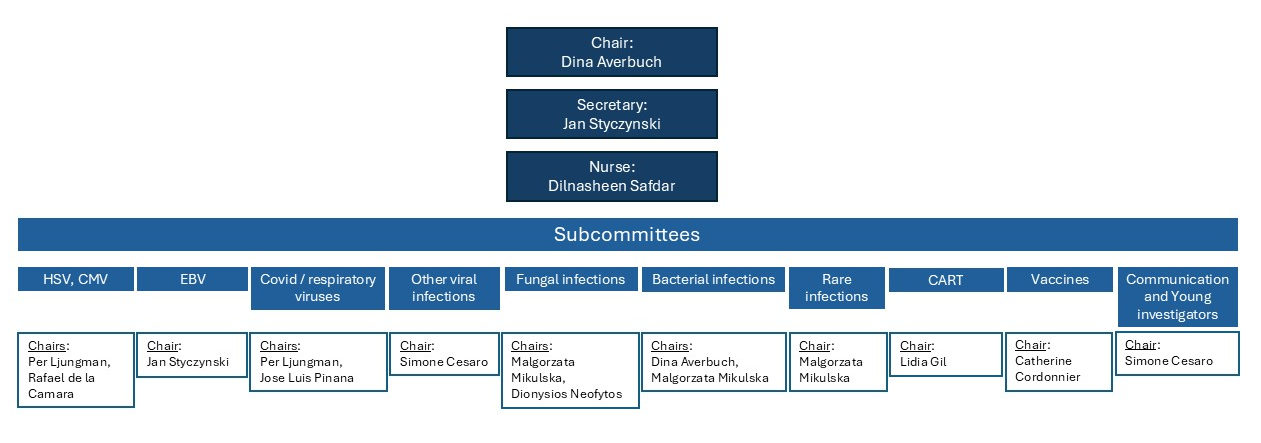
Structure
IDWP Organigram
EBMT Study Proposal and Selection Process Policy
Open Surveys
IDWP Publications List
Guidelines, Consensus Statements, and Position Papers
ECIL
The IDWP is one of the four participating bodies to the European Conference on Infections in Leukaemia (ECIL), along with the EORTC Infectious Diseases Group, the Supportive Care Group of the ELN, and the Immunocompromised Host Society.






