European Transplant and Cellular Therapy Online Registry (EuroTraCTOR)
EuroTraCTOR is an EU grant that was funded under the EU4Health programme 2021-2027 (grant number 101079887). The objective of the project is to design, develop and implement a new EBMT registry to improve the processes of collecting and using data across EU health systems. EBMT is a coordinating entity and counts with the support from other 10 project beneficiaries and 4 external expert organisations. The project started at the end of 2022 and has a duration of 30 months.
About the project
The constant evolution of Hematopoietic Cell Transplantation (HCT) and cellular therapies requires the continuous development and improvement of information technology (IT) tools and features, as a prerequisite to adjust to new and innovative treatments and associated legal and technical requirements. In order to answer the current and future needs of the HCT stakeholders –Professionals and Competent Authorities (CA) – the EBMT Registry requires a significant update of its structure and features.
The proposed development will enable the correct monitoring of HCT activities, patient and donor safety oversight, and recording/collecting quality, safety, and efficacy data. A new EBMT registry will be built based on the needs identified by HCT professionals and CA and aims to be as flexible as possible, allowing its customization at regional/federal levels to serve and adapt to different realities, in a user-friendly manner.
The future platform aims to provide an adequate answer to the requirements of clinicians/professionals involved in HCT and Cellular Therapies on one hand and other stakeholders such as CA or other health authorities on the other while complying with best practices associated with standardization and data protection (General Data Protection Regulation - GDPR), ultimately enabling better health decisions leading to improved, outcomes and care for EU.
Grant details
- Call topic:
EU4H-2021-PJ-17: Action grants to organise and collect data to understand the safety, quality and efficacy of therapies applied in the field of assisted reproduction and based on haematopoietic stem cells - Budget: 3,3M € (2M € EU funding)
- Duration: 30 months
- Consortium: 11 beneficiaries; 4 external expert entities
- Coordinator: EBMT
Consortium
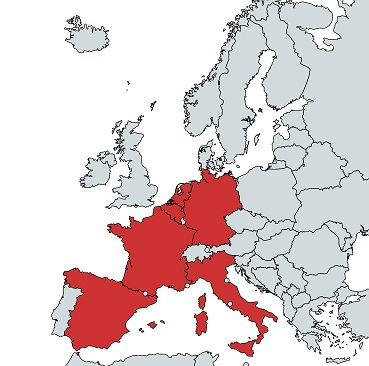
| Country | Short name | Name |
|---|---|---|
| NL | EBMT | European Society for Blood and Marrow Transplantation |
| ES | ONT | Organización Nacional de Trasplantes |
| IT | ISS-CNT | Istituto Superiore di Sanità - Centro Nazionale Trapianti |
| DE | DRST | Deutsches Register für hämatopoetische Stammzelltransplantation und Zelltherapie |
| DE | UKE | Interdisciplinary Clinic and Polyclinic for Stem Cell Transplantation at the University Medical Center Hamburg-Eppendorf |
| IT | GITMO | Gruppo Italiano Trapianto Di Midollo Osseo |
| FR | ABM | Agence de la Biomédecine |
| FR | SFGM-TC | Francophone Society of Bone Marrow Transplantation and Cellular Therapy |
| NL | RADBOUDUMC | Radboud University Medical Center |
| ES | GETH | Spanish Group of Hematopoietic Transplantation and Cell Therapy |
| BE | BCR | Belgian Cancer Registry |
External experts:
| Country | Short name | Name |
|---|---|---|
| EU | EDQM | European Directorate for the Quality of Medicines & HealthCare |
| NL | ZIN | The National Health Care Institute - Zorginstituut Nederland |
| CH | SBST | Swiss Blood Stem Cell Transplantation |
| UK | BSBMTCT | British Society of Blood and Marrow Transplantation and Cellular Therapy |
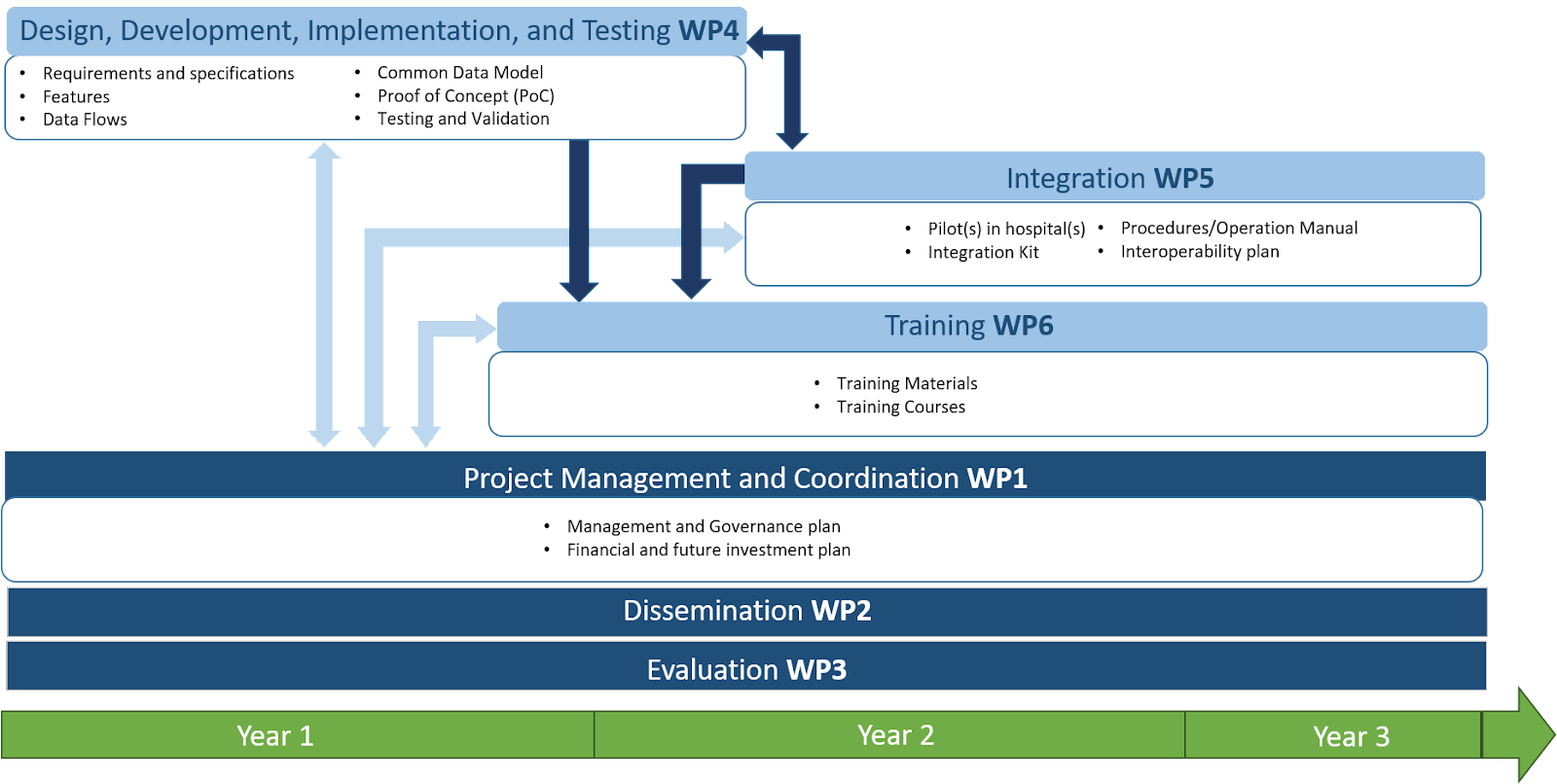
Project plan