As we move forward into the festive Christmas period, we would like to take this opportunity to remind you to continue reporting all CAR-T patients into ProMIse. If you have not yet started then please do so immediately.
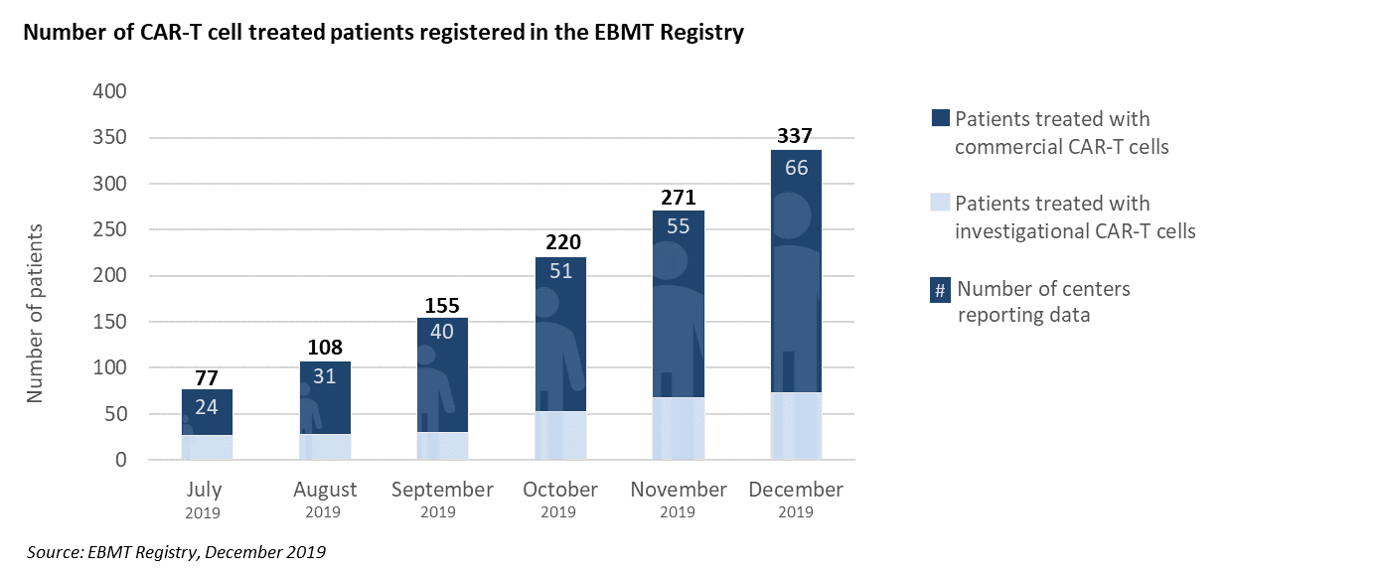
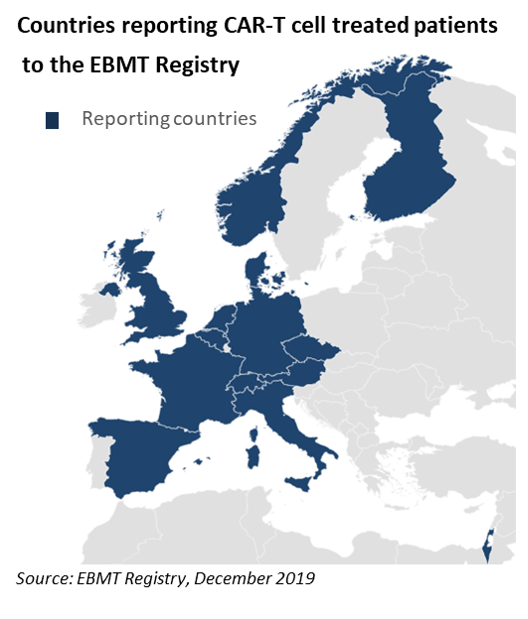
Across Europe and beyond the EBMT has started to capture CAR-T cell activity and as these patient numbers begin to increase it remains paramount that their safety is closely monitored.
We thank you all for your commitment and contributions as we move into this exciting phase of our work.
Please allocate sufficient time when ordering the medicinal product to receiving it at the hospital ready for administration to your patient.
It is important to understand that there will be a proportion of patients who are unable to receive the treatment due to reasons including progression of disease.
We therefore ask you to register your candidate patients into ProMIse as soon as the drug is ordered from the company, usually by your hospital pharmacy.
Thank you for your cooperation.
The EBMT Registry team