Advances in basic science, technology and medicine continually create opportunities for new and improved Tissue and Cells Therapies and Products (TCTP). These may be wholly new types, or improved methodologies for the preparation of existing TCTPs. While the objective of these changes and novelties is to prepare TCTPs that are safer, clinically more effective and meet the needs of clinicians and patients, there is always a risk that any change in the processing method can result in harm in the recipient. It is therefore vital that an evaluation of the potential risk of a process is systematically evaluated whenever a significant change is made.
When dealing with novelties in stem cell therapy, often life-saving product innovations are involved. It is for the best of the patients, that the process of setting conditions under which clinical use can be considered, proceeds seamlessly, not only to safeguard the quality and safety of these novel therapies, but also to facilitate the approval process for clinical use.
The EuroGTP II project (European Union (EU)-funded project, 2015-2019) aimed at establishing good practices with regard to Tissues and Cells (T&C) with a special focus on the risk assessment associated to the introduction of novel tissue / cell applications.
The project set up good practices governing tissue and cell preparation processes, and patient follow-up procedures in a safe and effective manner. This is pursued through the development of standardized methodologies (EuroGTP II Guide) and an interactive assessment tool (IAT).
The EuroGTP II methodologies gives the possibility to professionals to organize their risk assessments in a consistent manner and thus allows them to facilitate exchanges in novel therapies and treatments. The interactive tool takes care of the entire process of risk-based thinking and will result in uniform and in-depth risk analyses concerning novelties. Moreover, the Euro GTP II risk assessment tool can demonstrate that adequate in vitro, in vivo studies and clinical follow up programs are designed for these innovative treatments, and thus compliantly bridges the gap between science and clinical practice.
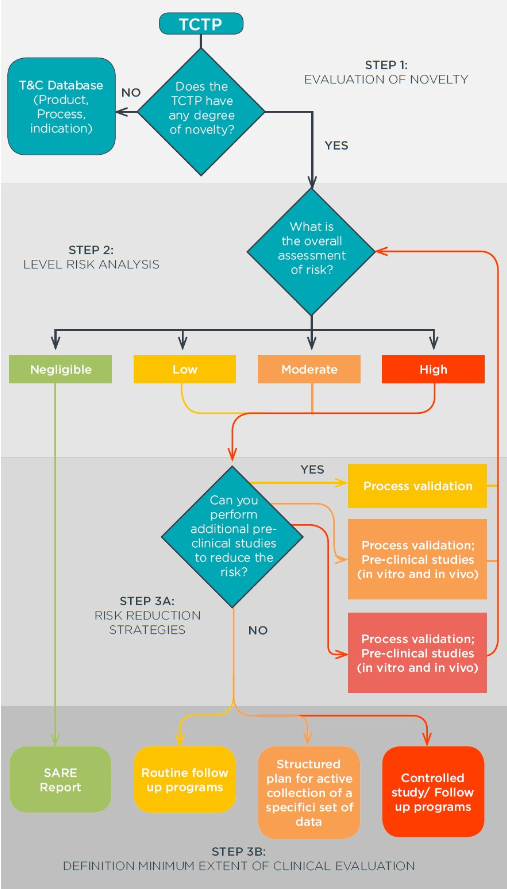
While the follow-ups of novel HSC therapies should aligned as much as possible with the registries and definitions of the EBMT, which are already the standard for the majority of European transplant centres, the EuroGTP II provides standard methodologies to evaluate novelty (Step 1), identify and quantify the potential risks of that given innovation (Step 2), and plan the extent of studies needed on the basis of the risks quantified (Step 3).
The EBMT was one of the collaborative partners of this project, who contributed for the definition of specific risk factors and risk consequences associated with HSC therapies. Currently, EBMT is also part of the EuroGTP II Management Committee, which aims to ensure the constant update and improvement of the project’s outcomes.
You can now find the EuroGTP II guide an Interactive Assessment Tool at the following link http://www.goodtissuepractices.site
You can also enjoy and enrol for free on the EuroGTP II e-learning course! The course allows participants to improve the scientific assessment of novel products/processes/clinical indications and associated risks, and promote the correct definition of evaluation protocols to guarantee its safe implementation.