Registry/Data Management
Cellular Therapy & Immunobiology Working Party (CTIWP)

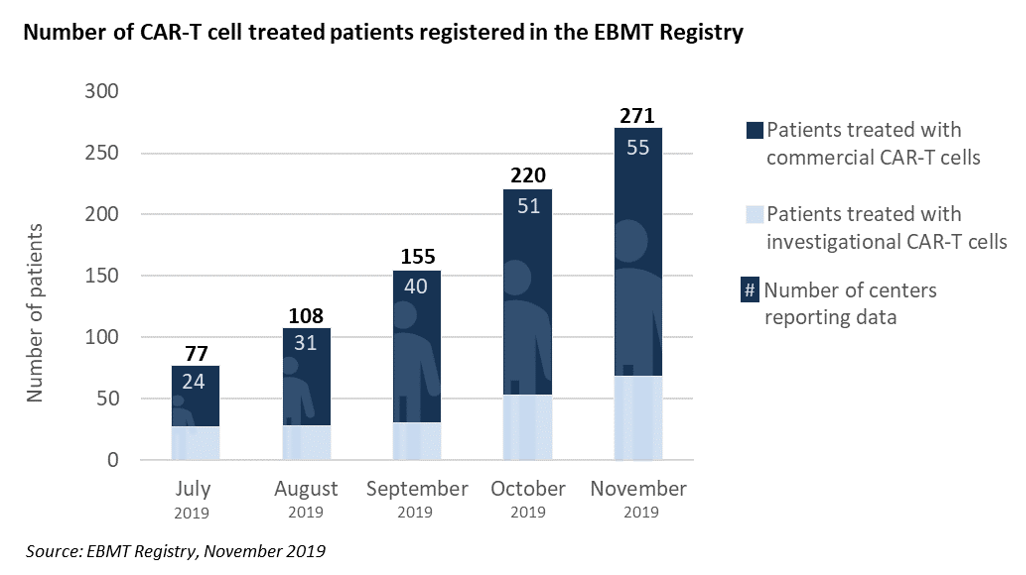
EBMT centre members and national registries are requested to document CAR-T cell therapies
This is part of a long-term effort and commitment of EBMT, together with all interested stakeholders, to contribute to a full assessment of the medical value of this new category of medicinal products, including long-term safety and efficacy. Read the September News HERE.