JACIE Activity Report 2022

Standards and Accreditation – JACIE

JACIE - The Joint Accreditation Committee ISCT-Europe & EBMT (JACIE) was established in 1998. It promotes high-quality patient care and laboratory performance in the collection, processing and administration of cellular therapy through a professionled, voluntary accreditation scheme. Since 2000, more than 500 Transplant programmes and facilities in 34 countries in Europe and beyond have applied resulting in over 800 initial and reaccreditations.
The inspections are carried out by the trained, volunteer inspectors who share their professional expertise to inspect against the Clinical, Collection, Processing and Quality Management Standards. Currently, there are approximately 300 inspectors across Europe, Middle East and Latin America. November 2022 saw a return of the Inspector training programme. The training model now includes e-learning modules for the theorical content with one day workshop focussing on developing the best practice for the inspection itself including pre-inspection preparation and post-inspection reporting.

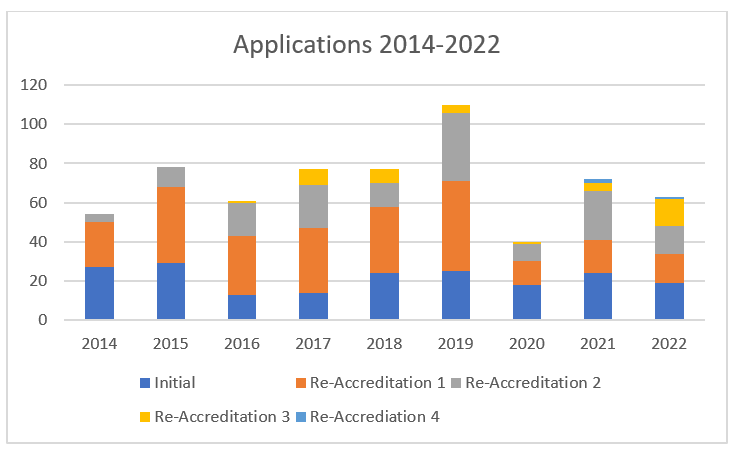
Applications
The number of applications has remained relatively stable with the exception of 2020 with lower than average number of applications due to Covid -19 pandemic.
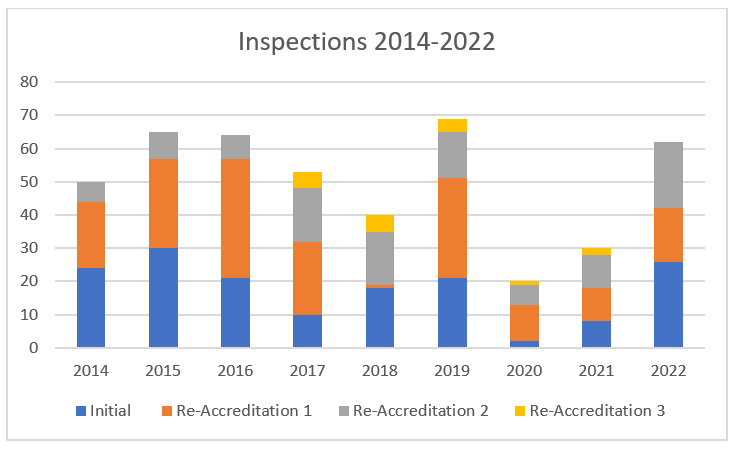
Inspections
2022 marked a return to nearly post-pandemic levels of inspection activity using all inspection modalities available (‘short process’, ‘remote’ and onsite inspections). In total, 64 inspections were carried out in 2022 (30 in 2021) and 26 centres achieved JACIE accreditation. Two key challenges for scheduling inspections has been centre preparedness and the inspector availability. Where possible, remote, even ‘hybrid’ (one of the inspectors carrying out the inspection remotely with the rest of the team onsite) forms have been adopted to facilitate the inspections where possible. However, many country-specific challenges remain due to requirements to meet language or other requirements or with the skill -mix of the active inspectors not matching the Centres waiting for inspections.
There is currently a total of 214 programmes in process as summarised in the table below.
| Total number of active facilities | 214 |
| Number of facilities in pre-inspection phase | 82 |
| Facilities ready to be inspected | 50 (18 with inspection dates confirmed) |
| Report awaited/being finalised | 31 |
| With the Accreditation Committee | 2 |
| Facilities Correcting Deficiencies | 49 |
Accreditations
In 2022, a total of 26 (28 in 2021) facilities were awarded JACIE Accreditation. We acknowledge the amount of work and commitment achieving the accreditation requires from the Facilities and we offer our congratulations to all the teams achieving this in 2022. These Institutions are listed below.
Institutions accredited in 2022
| Facility | Institution | City | Country |
|---|---|---|---|
| Stem Cell & Immunotherapies Laboratories | NHS Blood and Transplant | Liverpool | United Kingdom |
| Unidad de Trasplante Hematopoyético | Hospital Universitario Son Espases | Palma de Mallorca | Spain |
| Directorate of Clinical Haematology | Imperial College Healthcare NHS Trust | London | United Kingdom |
| Clínica Universidad de Navarra | Pamplona | Spain | |
| Programa de Transplantaçao | Instituto Português de Oncologia Porto Francisco Gentil, E.P.E. | Porto | Portugal |
| Stem Cell Laboratory | Manchester University Hospitals NHS Foundation Trust | Manchester | United Kingdom |
| Programma Centro Trapianti CSE Piacenza | Ospedale G. da Saliceto | Piacenza | Italy |
| Royal Manchester Children's Hospital | Manchester University Hospitals NHS Foundation Trust | Manchester | United Kingdom |
| Royal United Hospital Bath | Royal United Hospitals Bath NHS Foundation Trust (RUH) | Bath | United Kingdom |
| Dipartimento di Onco-emaologia e terapia cellulare genica | Ospedale Pediatrico Bambino Gesu | Rome | Italy |
| Hematologie et Therapie Cellulaire, Laboratoire de thérapie cellulaire | CHU Amiens Picardie | Amiens | France |
| The Christie Cellular Therapy and Transplant programme | The Christie NHS Foundation Trust | Manchester | United Kingdom |
| Programma Trapianti e Terapie Cellulari Fondazione | IRCCS Casa Sollievo della Sofferenza | San Giovanni Rotondo | Italy |
| Churchill Hospital - Autologous & Allogeneic Transplantation in Adult Patients & Immune Effector Cells Administration; Stoke Mandeville Hospital - Autologous Transplantation in Adult Patients; Royal Berkshire Hospital - Autologous Transplantation in Adult Patients |
Oxford University Hospitals NHS Foundation Trust | Oxford | United Kingdom |
| Hematopoietic Stem Cell Transplantation | Istituto G. Gaslini | Genova | Italy |
| Baskent University Adana Adult Bone Marrow Transplantation Center | Baskent University Adana Dr. Turgut Noyan Research and Medical Center | Adana | Turkey |
| Berlin Buch | Helios Klinikum Berlin-Buch | Berlin | Germany |
| Programma Trapianto Terapie Cellulari Ematologia Asugi Trieste | Azienda Sanitaria Universitaria Giuliano Isontina | Trieste | Italy |
| Stem Cell Transplantation Programme | Medisch Spectrum Twente | Enschede | Netherlands |
| Division of Stem Cell Transplantation and Immunotherapy, Department for Internal Medicine II, Hematology and Oncology | University Hospital Schleswig-Holstein, Campus Kiel | Kiel | Germany |
| Centre of Hematopoietic Cell Transplantation, Section of Hematology and Coagulation, Department of Medicine, Sahlgrenska University Hospital | Section of Haematology and Coagulation, Department of Medicine, Sahlgrenska University Hospital | Göteborg | Sweden |
| Sweden Centre of Hematopoietic Stem Cell Transplantation - Stem Cell Laboratory | Clinical Immunology and Transfusion Medicine, Sahlgrenska University, Hospital | Göteborg | Sweden |
| Stem Cell Transplantation Programme - Hematology | Universitair Ziekenhuis Brussel | Brussels | Belgium |
| Universitair Medisch Centrum Groningen | Groningen | Netherlands | |
| BST Barcelona | Banc de Sang i Teixits | Barcelona | Spain |
| BST - Hospital Mutua de Terrassa | Banc de Sang i Teixits | Terrassa | Spain |
Full list available at www.ebmt.org/jacie-accredited-centres
Benchmarking
JACIE continues to engage with the EBMT Benchmarking Reports although the operational management of this projects is with the Registry team. The centres participating in the project received their report in September 2022. A further manuscript on Benchmarking Project was submitted at the end of 2022.
Governance
Dr Kim Orchard (Southampton, UK) stepped down as the Chair of the JACIE Committee in August 2022; Committee which he had chaired since mid-2020. Dr Orchard, who has been an Inspector since 2004, continued to inspect regularly in the UK, Europe and further afield until his retirement from JACIE. He also served as the UK National Representative and as the Chair of the JACIE Accreditation Committee. JACIE remains hugely indebted to Dr Orchard for his commitment and leadership over his years of service to JACIE.
Following a search process, the Board approved Dr Lynn Manson (Edinburgh, UK) as the new Chair of the JACIE Committee in January 2023. Dr Manson will take over her new role with immediate effect. Dr Manson trained as a JACIE Inspector in 2010 and is a Collection and Quality Management Inspector. She has been a member of the Accreditation Committee since 2016 and a Chair of the Accreditation Committee since October 2020. In this role, she has provided valuable support to the JACIE Office resolving issues arising through the accreditation process actively contributing in this role to both JACIE and the Inspector Committees. Dr Manson has also been part of the FACT-JACIE Standards Sub-Committee for Collection for the 6th and 8th Editions of JACIE Standards.
JACIE Committees


JACIE Accreditation Committee in 2022
Lynn Manson - Accreditation Committee Chair
After a relatively quiet 2021 with the inspection activity recommencing in the latter part of 2021, the Accreditation Committee also recommended activity in full. In 2022 the JAC met twice a month with a total of 20 meetings (only 4 meetings in 2021). These teleconferences are scheduled for an hour at the time.
The Committee continued to assess onsite, remote and short process inspections. Chair of the Committee, Dr Manson, also continued to represent JAC in the Inspector Committee as well as the JACIE Committee providing JAC feedback in these two fora.
Dr Manson and JAC members have also actively participated as speakers on the Inspector Webinar programme.
Total of 26 centres were accredited in 2022– see the list above.
JACIE Inspector Committee in 2022
Eugenia Trigoso Arjona - Inspector Committee Chair
2022 was a very special year for the Inspector Committee, some old friends stepped down but new ones joined our Committee: Olga Lopez (Spain); Dania Arabi (Saudi Arabia); Fabio Culurgioni (Italy)
Following our working structure, the committee members decided to continue with our monthly meetings, so eleven meetings were scheduled for 2022, including the suggested topics:
- The Inspector training that were stopped during the COVID-19 pandemic should be resumed;
- The classification of the standards into 3 categories: standards specific for transplants, IEC and generic. This was already done for the 7th edition; but needs to be verified and validated;
- The classification of the criticality or the impact of non-compliance with the standards: this could be very useful in supporting any rethinking about the way inspections are done and could be helpful with establishing a more risk-based or focused reaccreditation process. It can bring attention to the critical areas that centres have to address.
Some of these topics have been achieved, others continue for 2023.
JIC face to face meeting
Taking advantage of the inspector training events, a F2F meeting was organised on Saturday 19th November 2022. The agenda items included: developing the 2023 agenda, objectives, and a work plan as a group, which includes participation in the new Inspectors Handbook and continuing to organise further training events, webinars etc. to help new inspectors in particular.
The JIC members have participated in the initial training for 20 candidates.
Webinars
Focussed on tricky standards and difficult moments of the Centers Inspections in both remote and on-site inspections. 4 webinars took place throughout the year 2022:
27/01 - Role of the Team Leader: 54 attendees
28/04 - Immune Effector Cells: 40 attendees
29/09 - CAR-T Cells for Inspectors: 29 attendees
24/11 - Onsite vs Remote Inspections: 39 attendees
All of them are available at: https://www.ebmt.org/jacie-inspectors-webinars
In order to prepare 9th ed. standards, a survey regarding the feedback about the 8th ed standards was circulated.
JACIE Quality Managers Committee in 2022
Renza Monteleone - Quality Managers Committee Chair
The Quality Managers Committee is a point of reference for quality managers working on stem Cells Transplant Program affiliated with EBMT, giving advice and support at any stage of the accreditation process.
In 2022, the Quality Managers Committee was composed by 9 members, experienced quality managers or responsible for quality management working in accredited centres or towards accreditation:
- Renza Monteleone (Reggio Calabria, Italy)
- Olga López (Madrid, ES)
- Dieter Klarmann (Frankfurt, DE)
- Ilknur Kozanoglu (Adana, TR)
- Anne Emmet (London, UK)
- Julie Dolva (Oslo, Norway)
- Songul Durmaz (Adana, TR)
- Phuong Huynh (Brussels, B)
- Mara Magri (Bergamo, Italy)
After the publication of the book, “Quality Management and Accreditation in Hematopoietic Stem Cell Transplantation and Cellular Therapy: The JACIE Guide”, the members of Quality Managers Committee, authors in several chapters, have been constantly available to reply to any questions and doubts related to the different aspects of quality management system and accreditation.
As every year, the Quality Managers Committee worked on planning the 15th Meeting of the Quality Management Group at the 49th Annual Meeting of the EBMT in 2023, with several online meetings to draft the program, to decide the topic and to select the speakers.
The Quality Managers Committee actively collaborates with the JACIE Office, JACIE Accreditation Committee and Inspectors Committee, to create training courses and resources that are helpful for the Quality Managers.
