JACIE Activity Report 2021


Standards and Accreditation – JACIE

The Joint Accreditation Committee ISCT-Europe & EBMT (JACIE) was established in 1998. It promotes high-quality patient care and laboratory performance in the collection, processing and administration of cellular therapy through a profession-led, voluntary accreditation scheme.
JACIE works continuously with international partner organisations to develop and maintain standards for the provision of quality medical and laboratory practice in HSCT, performs on-site inspections, and accredits those programmes that demonstrate compliance with these standards. JACIE also provides training for inspectors and centres on the accreditation process.
Since 2000, 508 transplant programmes and facilities in 34 countries in Europe and beyond have applied to JACIE and 790 inspections (first-time and reaccreditation) have been performed. 368 applicants have achieved accreditation at least once with practically all centres repeating the process after the first accreditation cycle. There are over 300 registered inspectors, all volunteers drawn from the HSCT and cellular therapy field.
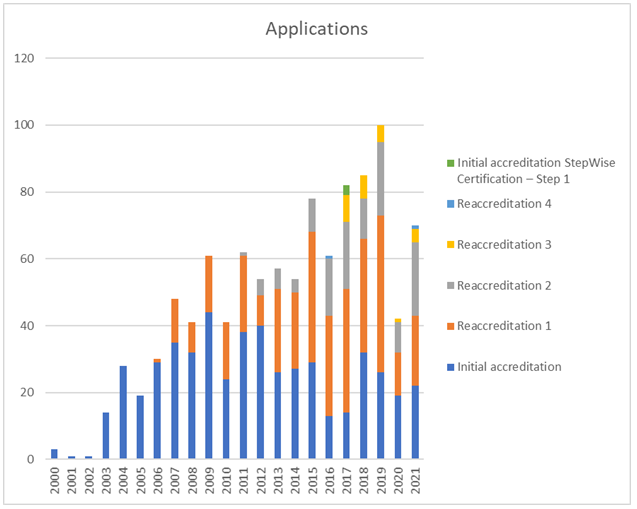
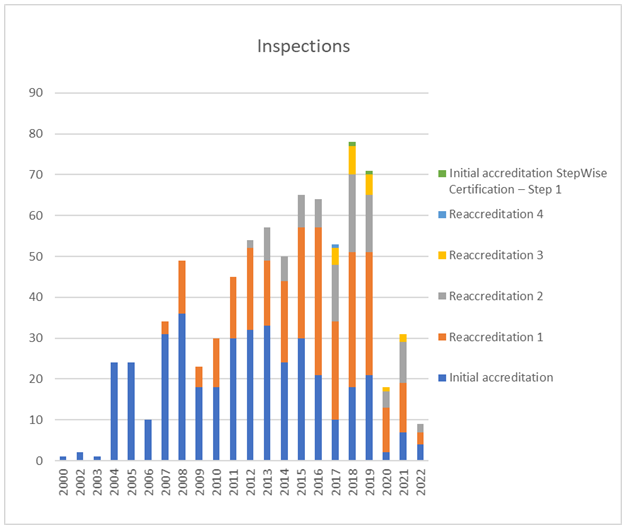
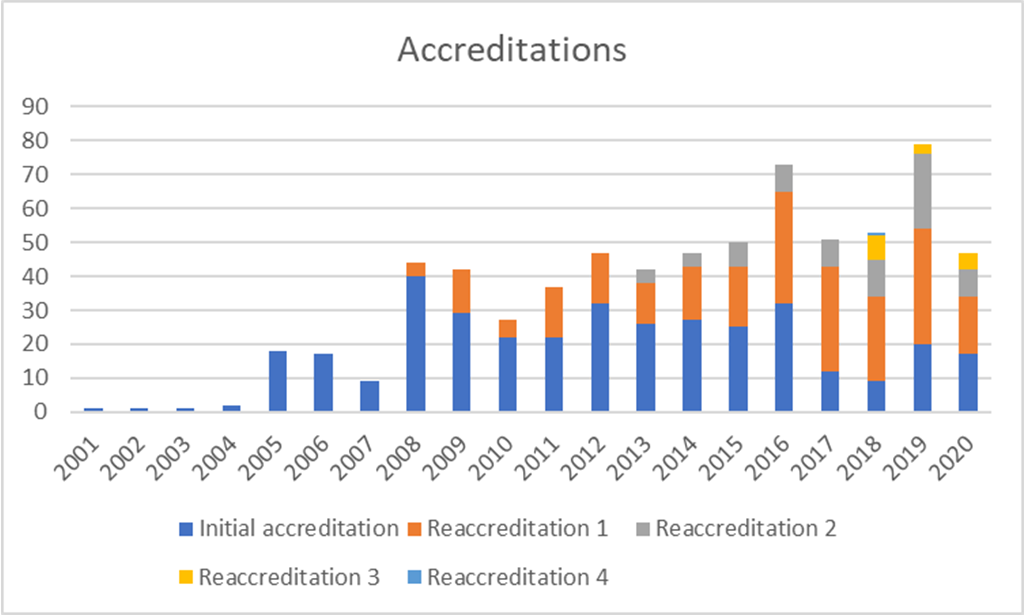
In terms of activity, 2021 continued to see severe negative impact of the pandemic on activities although this has been somewhat mitigated by the introduction of a remote inspection format and a so-called Short Process which avoid the need for inspectors to travel. 70 applications were received, 31 inspections of different formats were performed and 28 accreditations were awarded.
Applications
Inspections
Accreditations
Benchmarking
The second benchmarking exercise was performed in July 2021. A total of 395 centres were eligible to participate in the 2021 exercise. Of the notified centres, 68% (260 centres) were able to pick up the report: 200 directly through Google Drive access, 36 through their national registries and 24 were sent directly to the centres that were not able to access the drive themselves.
The majority (95%) of the centres that evaluated the report found the content comprehensible. 98% evaluated the quality as good and considered the current report an improvement on the previous release/version.
8th Edition of FACT-JACIE Standards
The 8th edition of the FACT-JACIE Standards was published in May 2021.
Other
The JACIE Twitter account @JACIE_EBMT has grown to 1,789 followers.
We would like to express our continued appreciation and admiration for the Inspectors, JACIE Committee Members, Accreditation, Standards and Quality Manager Committee members, other volunteers and the JACIE Office team for all their tremendous hard work, commitment and dedication.
Institutions awarded accreditation in 2021
- Hospital Privado Universitario de Córdoba, Córdoba, Argentina
- Institut Jules Bordet, Brussels, Belgium
- CHU Haut-Levêque, Pessac (Bordeaux), France
- Bayerische Stammzellbank gGmbH, Gauting, Germany
- Tel Aviv Sourasky Medical Centre, Tel Aviv, Israel
- Istituto L.e A. Seràgnoli, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy
- Ospedale Civile di Civitanova Marche Area Vasta 3 Marche, Civitanova Marche, Italy
- Ospedale Policlinico San Martino, Genova, Italy
- Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy
- Azienda Unità Sanitaria Locale IRCCS Reggio Emilia, Reggio Emilia, Italy
- Rome Transplant Network, Policlinico Tor Vergata, Rome, Italy
- Humanitas Cancer Center, Rozzano, Italy
- AOU Integrata Verona, Verona, Italy
- King Faisal Specialist Hospital, Riyadh, Saudi Arabia
- Hospital Universitario de la Paz, Madrid, Spain
- Hospital Universitario Puerta de Hierro Majadahonda, Madrid, Spain
- Hospital Universitario y Politécnico de La Fe, Valencia, Spain
- University Hospital, Linköping, Sweden
- Kantonsspital Aarau AG, Aarau, Switzerland
- Universitätsspital Basel, Basel, Switzerland
- Oncology Institute of Southern Switzerland (IOSI), Bellinzona, Switzerland
- Universitätspital Zürich, Zürich, Switzerland
- NHS Blood and Transplant, Barnsley, United Kingdom
- University Hospital of Wales, Cardiff and Vale University Health Board and Singleton Hospital, Abertawe Bro Morgannwg University Health Board, Cardiff/Swansea, United Kingdom
- Leeds Teaching Hospitals NHS Trust, Mid Yorkshire NHS Trust, NHS Blood and Transplant Leeds, Leeds, United Kingdom
- Alder Hey NHS Foundation Trust, Liverpool, United Kingdom
- NHS Blood and Transplant, Liverpool, United Kingdom
- Biovault Technical Ltd, Plymouth, United Kingdom
Full list available at www.ebmt.org/jacie-accredited-centres
Governance
Dr. Kim Orchard (Southampton, UK) succeeded Prof. John Snowden as Chair of the JACIE Committee in mid-2020. Prof. Snowden, now the EBMT Secretary, remains on the Committee. See https://www.ebmt.org/jacie-committee for more details.
Dr. Isabel Sánchez Ortega joined EBMT in March 2020 as Medical Officer. Among her responsibilities is supporting JACIE. Dr. Sánchez Ortega took over the JACIE Medical Director duties from Dr. Riccardo Saccardi, who occupied this role from January 2016 to June 2020.
Quality Managers Committee
The Quality Managers Committee was established in 2011. All of the members are experienced quality managers or responsible for quality management working in accredited centres or towards accreditation. The Committee is chaired by Ms. Renza Monteleone (Reggio Calabria, IT). See https://www.ebmt.org/quality-managers-committee for more details.

JACIE Accreditation Committee - Activity Report 2021
2021 saw a significant downturn in the activity of the JACIE Accreditation Committee from its previous activity level of 2 teleconferences per month, commensurate with the cessation of on-site inspections of stem cell transplant programmes as a result of the COVID pandemic.
Four teleconferences were undertaken:
18/01/2021 - Pavia, Italy - On-site
16/08/2021 -
Manchester, UK - Pilot: remote
Rome, Italy Pilot: remote
20/09/2021 - Creteil, France- Pilot: remote
| Centre | Inspection Round | Areas Inspected |
|---|---|---|
| Pavia, Italy | Reaccreditation 1 | Clinical paediatric, BM paediatric and apheresis collection (due inspection in2022: clinical adult+IEC, BM adults collection, processing). |
| Manchester, UK | Reaccreditation 2 | Processing |
| Rome, Italy | Reaccreditation1 | Clinical paediatric (auto/allo) + IEC, BM and apheresis collection |
| Creteil, France | Reaccreditation 2 | Clinical adult (allo)+IEC, BM and apheresis collection, processing |
Reports from the three pilot inspections using the new remote inspection process were reviewed at the two teleconferences in August / September.
A further teleconference is scheduled for mid-December dependent upon availability of the inspection report which will be the first desk-top documentation review of a Centre assessed to be low risk (and so eligible for documentation review only) as per the Short (inspection) Process.
See https://www.ebmt.org/accreditation-committee for more details.

