
Plenary sessions
Sickle cell disease - haploidentical transplantation or gene therapy: what is the future?
Sickle cell disease (SCD) remains an inherited disorder with substantial morbidity and mortality despite significant improvements in the supportive care and primary prophylaxis. Although curable via allogeneic HSCT, donor availability has limited frequency. Most recently, therapeutic options such as haploidentical HSCT have increasingly demonstrated a safe and effective alternative option to matched donor HSCT. A significant increase in transplant frequency for this indication is now expected. Additionally, gene therapy, one of the most exciting recent developments, has finally reached clinical stage.
Marina Cavazzana, one of the pioneers of viral based gene therapy, summarized in an eloquent presentation the state of the art of lentiviral based approaches to introduce manipulated hemoglobin B or F gene constructs or raise hemoglobin F levels in order to prevent sickling.
She was followed by Josu de la Fuente, who elucidated the field of conventional transplantation and the progression to available haploidentical HSCT strategies. He very convincingly showed that either post-transplant cyclophosphamide or T cell depleted approaches can offer cure, even for advanced stage SCD patients, with a low risk of rejection and GvHD, and excellent overall- and disease-free survival rates.
Last but not least, Matthew Porteus gave us insight into the future of gene editing. Whereas current options remain surrogate therapies, SCD is the protagonist for proper gene editing via homologous recombination of a single nucleotide mutation. Still in preclinical stage, utmost elegant treatment options are emerging that might eventually be able to fully correct SCD.
Click HERE to view the programme and presentations.
And watch the EBMT TV debate “Cell Therapy in Children”
New developments in the field of CAR cell therapy
Less than two months after the 1st and enormously successful European Symposium on CAR-T Cells, co-organized in Paris by the EBMT and the EHA, the 45th Annual Meeting of the EBMT held in Frankfurt was again a “hot spot” for those interested in the development of this new category of medicinal products, and more broadly of immune effector cell therapies. The plenary session held on Tuesday, March 26th included several presentations that illustrate several trends in the field.
Two approved and industry-manufactured drug products are now available to European practitioners; EU hospitals and healthcare authorities are gearing up to offer their patients safe and equitable access to these autologous CAR-T cells targeting CD19. However, these two gene therapy medicinal products only cover a small proportion of the needs in onco-hematology.
At EBMT 2019, we were fortunate to hear one of the pioneers of the development of “T-bodies” – Pr Hinrich Abken (University of Regensburg, Germany) - explained how CAR-T cells can be further engineered with the goal to counteract the immunosuppressive environment present in many cancers, and thus extend the use of CAR-T cells to other hematological malignancies but mostly to solid tumors. Dr Michael Hudecek (University of Würzburg, Germany) provided an overview of the manufacturing process for autologous CAR-T Cells, and illustrated how academic EU institutions – some of them networked through EU supported consortium - now develop new drug products targeting different antigens and diseases, and compete with north-American or Chinese institutions. Pr Persis Amrolia (Great Ormond Street Hospital, London, UK) presented the results of an elegant early academia-industry clinical trial evaluating how a bispecific autologous CAR-T cell simultaneously targeting CD19 and CD22 can tackle the most prevalent mechanism of resistance to CD19 autologous CAR-T Cells, i.e. the loss of CD19 expression: 10 children or young adults with high-risk ALL were treated; although the follow-up is short, all patients but one presented with molecular remission at one month, and none of the observed relapses were associated with the loss of CD19. Finally, Pr Kathy Rezvani illustrated that Chimeric Antigen Receptors can “team up” not only with T-Cells, but also with other immune effector cells such as Natural Killer cells. Pr Rezvani presented the results of early clinical trials with allogeneic off-the-shelf “CAR-NK Cells” manufactured from cord blood units, and targeting CD19 positive hematological malignancies, suggesting efficacy associated with a favorable safety profile, notably the lack of cytokine release syndrome (CRS). She concluded with a brief overview of the programs in development at MD Anderson Cancer Research Center that take advantage of this technology, and again extend beyond hematology into the field of medical oncology.
Click HERE to view the programme and presentations.
And watch the EBMT TV debate “CAR-T Cell Therapy: New Hope for Patients?”
Relapse after transplant for acute leukaemia
The improvements achieved over the last decade in the prevention and treatment of transplant-related complications have exposed disease relapse as the main obstacle that should be overcome in order to fully exploit the therapeutic potential of allogeneic HSCT.
The four presentations from this Plenary Session focused on the latest research findings on the biology that govern post-transplantation relapse, and on how to translate this knowledge into new therapeutic rationales.
Dr. Luca Vago from the San Raffaele Scientific Institute, Milano, Italy, illustrated the genomic and non-genomic changes that occur in acute myeloid leukemia (AML) cells under the selective pressure mediated by the donor immune system, leading to the selection of immune resistant relapse variants.
Prof. Roland Meisel from Heinrich-Heine-University, Düsseldorf, Germany, presented a detailed analysis of the mutational landscape of childhood acute lymphoblastic leukemia before and after transplantation, focusing on the dynamics of "druggable" mutations.
Prof. Paresh Vyas from Oxford University, Oxford, UK, detailed the dynamics of AML clonal evolution upon stem cell transplantation or targeted therapy with IDH2 inhibitor enasidenib, beautifully demonstrating how knowledge of the clonal structure of different hematopoietic subsets can translate in clinically-relevant indications on therapy response or resistance.
Finally, Prof. Jurge Finke, from the University of Freiburg, Freiburg, Germany, presented an overview of the different therapeutic options currently available for relapsed patients, including epigenetic therapy with demethylating agents, use of targeted agents such as FLT3 inhibitors or immune-based approaches such as donor lymphocyte infusion or second allogeneic transplantation.
Click HERE to view the programme and presentations.
Special Sessions
Transplant survival outcomes: achieving fair and acceptable benchmarking for the EBMT community
Benchmarking (defined as “the process of comparing a practice’s performance with an external standard”) will provide transplant professionals with a means to assess how their unit is performing compared to the wider BMT community and help centres address the JACIE standards relating to survival outcomes. Benchmarking of survival outcomes has been developed as an integral part of Project 2020. Ultimately, we hope that this will benefit patient care across EBMT by promoting corrective action in 'underperforming' centres whilst the BMT community learns from 'high-performing' centres.
The EBMT ‘Clinical Outcomes Group’ has made good progress towards development of ‘benchmarking’, which was summarised in the Special Session chaired by Riccardo Saccardi and John Snowden from JACIE. Kim Orchard and Francesca Bonifazi discussed current national models; Doug Rizzo summarised the success of the CIBMTR model; Myriam Labopin and Hein Putter appraised the statistical challenges and summarised the ‘first base’ statistical model developed for EBMT. Finally, John Snowden summed up the progress so far and what needs to be done before we can implement the model across EBMT and JACIE, and also how it can be sustained in the long term (i.e. ‘second base’ and beyond).
As mentioned above, the aim of this ‘work package’ of EBMT Project 2020 is to create a fair, risk adapted, acceptable, benchmarking process across EBMT centres, feasibly delivered via the Registry, to enable centres, to assess 1-year survival outcomes for both allogeneic and autologous HSCT. JACIE accreditation standards (e.g. B4.7.5), have been a key driver, but, of course, there are much broader implications, ranging from complete and accurate data reporting by centres to our professional responsibilities to act upon results, along with R&D to assess the global impacts we can make by implementing benchmarking across EBMT.
This is just the start. The first phase or ‘first base’ of the statistical model has been led by Hein Putter and Erik van Zwet, Leiden University Medical Centre, with further statistical input from Myriam Labopin, from the EBMT Statistical Committee, and clinical steer from the Clinical Outcomes Group. There will be ongoing refinement of the system over the next several years.
There are some immediate updates. It became very clear early in the project that one of the most significant determinants of results is the completeness of follow up, and it will be no surprise that the quality of follow up in the EBMT Registry varies significantly between centres. The current model for 1-year overall survival therefore includes only higher reporting centres. However, for all centres the Clinical Outcomes Group has chosen to prioritise improving the quality of follow up reporting with a similar benchmarking model for ‘data completeness’, which parallels the ‘funnel plot’ model for 1-year survival. We hope this will have an impact in alerting centres to optimising reporting of follow up, which in turn will improve the validity of and confidence in the 1-year survival benchmarking output and extend it across a broader number of centres.
Click HERE to view the programme and presentations.
And watch the EBMT TV debate “Quality and Survival Outcomes”
The present place of haploidentical transplants throughout the world
This EBMT Global Committee Special Session, chaired by Prof Nicolaus Kröger and Prof Norbert Claude Gorin, took place on Wednesday March 27 in Frankfurt. It explored the development of haploidentical transplantation (HAPLO) in China, Japan, Lebanon, India and Argentina.
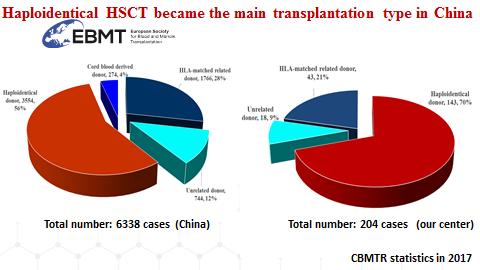
Outside the US, clearly, the largest experience with haploidentical transplantation in the world is presently in China. Professor Huang He from Zhejiang University in Hangzhou (China) presented a review of the Chinese experience and also focused on the experience of his own center. So far, of a total of 6638 allogeneic transplants reported to the Chinese registry during the year 2017, 3554 have been HAPLO, which represents 56% of all allogeneic transplants. The situation is the same in Hangzhou with 204 HAPLO representing 44% of all allogeneic transplants of this single center. The present activity in Hangzhou (year 2018) can be split into one third matched sibling donors (MSD), one third matched unrelated donors (URD) and one third HAPLO. MSD remain the first choice. Huang He showed that the relapse incidence post HAPLO was lower than with MSD or URD especially in high risk patients. HAPLO and CART cells presently are the main activity of the Hangzhou transplant center and combine with each other within therapeutic strategies such as CART cells to reach complete remission (CR) followed by HAPLO or CART cells to rescue patients in relapse post HAPLO. As an interesting example, Pr Huang He showed results in 53 patients with ALL in relapse or chemotherapy refractory (R/R) who received CD19 CAR T cells. Forty nine of them went into CR with no detectable minimal residual disease (MRD negative). Data in one patient showed that CAR-T cells penetrated blood-testis barrier and eradicated leukemia cells in testis. Nineteen patients then went to HAPLO.
The Chinese experience in general and the Hangzhou experience in particular is important for the transplant community worldwide and very precious for EBMT.
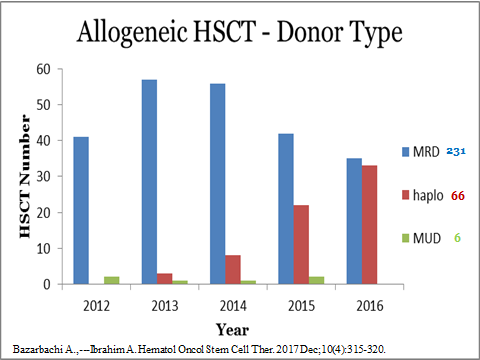
Professor Ali Bazarbachi (American University of Beirut, Lebanon) reported a growing HAPLO activity in Lebanon. Since 2015, 66 HAPLO were done in 64 patients, which include 21 transplants in 2018. The major indication was Acute Myeloid Leukemia (AML) in 30 patients and lymphomas in 16. TBF (Thiotepa, Busulfan, Fludarabin) was the preferred conditioning regimen. The majority of patients received peripheral blood stem cells. The Non relapse Mortality (NRM) at one year was 18%, the incidence of acute Graft versus Host Disease (aGVHD) grade III-IV 11% and the incidence of chronic GVHD 14%. In 16 patients with AML receiving an HAPLO in CR ( CR1=15, CR2=3), the progression free survival (PFS) and the overall survival (OS) were respectively 57% and 75% at three years, not different from the experience of this center with MSD retrospectively. Pr Ali Bazarbachi concluded that although haploidentical transplant was only recently introduced in the Middle East, it was rapidly becoming a major donor source of allogeneic stem cells.
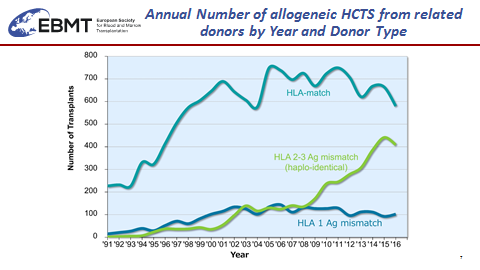
Professor Shinichiro Okamoto from Keio University School of Medicine and on behalf of the Japan Society for Hematopoietic Cell Transplantation (JSHCT) summarized the transplant activity in Japan and also other Asian Pacific countries. While unrelated cord blood (UCB) is largely used in Japan in adult patients and indeed competes with URD, HAPLO is booming up with more than 500 transplants per year. Likewise, during the year 2016 already more than 4000 HAPLO have been done in Asian Pacific countries. Pr Okamoto presented a Retrospective Comparison of URD versus related haplo HSCT in Japan. The HAPLO group consisted of 140 patients receiving post-transplant high dose cyclophosphamide (PTCY) following the Baltimore protocol for GVHD prevention. HAPLO was significantly associated in multivariate analysis with the lowest NRM, the lowest incidence of aGVHD and higher (but non-significant) incidence of relapse.
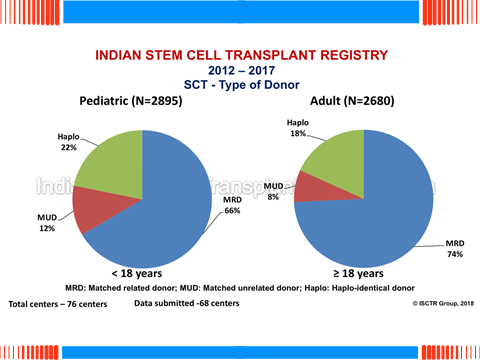
Professor Lalit Kumar from the Department of Medical Oncology (All India Institute of Medical Sciences, New Delhi, India) gave an impressive review of the India transplant registry as well as the experience of the CMC center in Vellore. The Indian stem cell transplant registry presently contains data on 14534 patients transplanted during the period from 1983 to2017. 8624 were allogeneic transplants of which 23% concerned patients with AML and 22% hemoglobin congenital disorders. Haploidentical donor transplants were done in 18% of adults and 22% of children. There were several issues, including the fact that, since most of the expenses are covered by the patients, the selection of the donor is also a matter of financial concern. Another issue was the prevalence of infectious diseases which made infection post transplantation an important post-transplant complication, with Acinetobacter, pseudomonas species, carbapenem resistance as well as fungal infection as major issues. Professor Kumar then reported the HAPLO experience from the Christian Medical College (CMC) in Vellore: 95 consecutive HAPLO were performed from 2010 to 2015 in 41 adults and 54 children mostly with fathers and mothers as stem cell donors. The therapeutic scheme copied the Baltimore approach with PTCY for GVHD prevention. The population of patients was heterogeneous and results were presented by risk scores. Early mortality and graft rejection unfortunately affected a total of 37 patients. The incidence of bacterial (44%), fungal (24%) and viral (53%) infections were high. Liver veino occlusive disease (VOD) and hemorrhagic cystitis occurred in 17% and 8% of the patients. As seen in most other EBMT centers, the incidence of grade III-IV acute GVHD (13%) and severe chronic GVHD (13%) was low. Professor Kumar concluded that Haplo-identical SCT is an encouraging option for patients requiring allogeneic SCT in the absence of a HLA-matched donor, but also that post-transplant infection in India was an important cause of death. His center is now aiming at better patient selection and earlier transplant to improve outcomes.
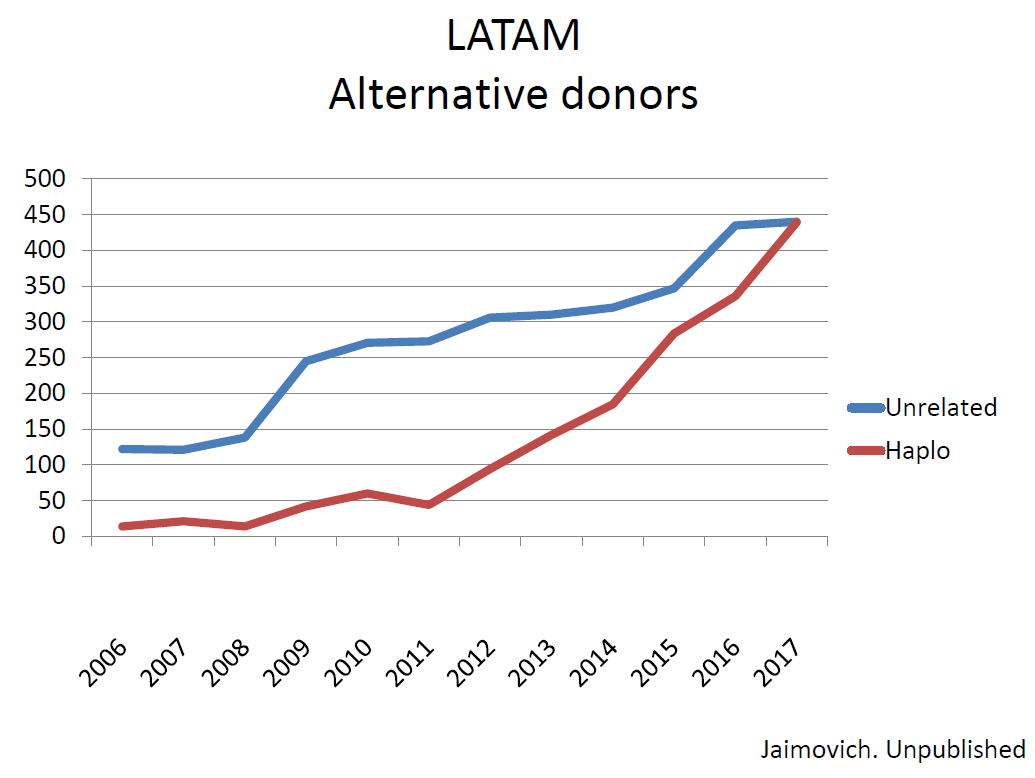
Professor Gregorio Jaimovich from the Favaloro University Hospital and Anchorena Hospital (Buenos Aires, Argentina) indicated for Latin America (LATAM) a total of about 2700 allogeneic stem cell transplantations done in 2017. HAPLO are currently done in Argentina, Brazil, Chile, Colombia and Mexico. Interestingly nowadays the number of HAPLO (mostly with PTCY) per year in LATAM has catched up the number of UD transplants. Gregorio Jaimovich showed the comparison recently reported by AL Basquiera et al in Argentina which showed similar outcomes after UD and HAPLO for adult ALL.
In summary the session, although taking place on the last day of the EBMT Annual Meeting, was very well attended. It did confirm that HAPLO is booming not only in pioneer expert centers but in fact everywhere in the world, including new emerging centers who quickly take advantage from the simplification brought by HAPLO, namely a reduction in cost, the ability to get a suitable donor fast and a reduction in the incidence and severity of acute and severe chronic GVHD, while preserving a strong GVL effect.
A second specific EGC session is already planned for the EBMT 2020 Annual Meeting in Madrid on the following topic: “Performing transplants in patients with infectious diseases”. Colleagues from outside Europe with experience in this field and wishing to share their experience in Madrid are welcome and should contact the EGC.
Click HERE to view the programme and presentations.
Visit the EBMT Global Committee page.
Social Media Session
The first talk by Mohamad Mohty focused on managing your virtual reputation: from a health care professional standpoint. He questioned the positive aspects of social media and highlighted some of the challenges, consequences and negative aspects of being on the cloud. He also provided some tips to help attendees consider how to manage their social media/online presence. Navneet Majhail delved into such topic as patient rating of physicians/healthcare providers and how this is done and used in the USA, and how it has become a metric that is looked at very closely. Orla Stewart and Gillian Adams then joined a panel discussion and addressed questions such as: where does patient rating of physicians stand in Europe and where will it go in the future? Are there country specific differences? What are some ways to manage your online reputation?
Click HERE to view the programme and presentations.
Best of EBMT 2019
The speakers of this session provided the main highlights in adults, cell and immune therapy and paediatrics.
Click HERE to view the programme and some of the presentations.