Scientific report 2020 of the Cellular Therapy and Immunobiology Working Party (CTIWP)

Major achievements
Although affected by the COVID-19 pandemic as everyone in the world, the CTIWP remained active in 2020.
Registrations of CAR-T Cells treated patients continued and reached the milestone of 1,000 patients in December. The CTIWP largely contributed to the production of the first reports for Post-Authorization Safety Studies sent to Novartis and Kite / Gilead, using aggregated data, while the CTO worked hard to establish contracts with CAR-T Cells treating center in order to establish conditions that allow secondary use of data and center compensation, with a view to further improve the quality and utility of collected data. Scientific priorities, data access, Cellular Therapy Form revisions, harmonization of center qualification criteria and processes are some of the topics and work packages included in the GoCART initiative that aims to bring all interested parties in the field of Immune Effector Cells – particularly CAR-T Cells – to join forces and cooperate in order to fully measure the medical value of these innovative therapies and remain competitive with our colleagues in the rest of the world
Meanwhile, the CTIWP continues to explore the field of autologous and allogeneic hematopoietic cell transplantation, and several studies were pursued or initiated in 2020.
Principal research studies
- A survey of immune monitoring practices for viral infections post-HSCT in Europe (L Vago & R Greco and others, Immune Monitoring Subcommittee). Collecting data.
- A study of immune reconstitution post allogeneic hematopoietic cell transplant (M Cavazzan, A Toubert, L Vago R Grecco, F Simonetta, J Kuball and others, Immune Monitoring Subcommittee). Collecting data.
- A study of the consequences of donor-recipient HLA matching on the outcome of allogeneic hematopoietic stem cell transplantation from unrelated donors (K Fleischhauer et al. Immunogenetics subcommittee, in collaboration with DKMZ). Manuscript in preparation.
- Evaluation of Killer Cell Immunoglobulin-like receptor HLA class I ligand models for donor selection (J Schettelig and others; Immunogenetics Subcommittee, I. collaboration with DKMZ and with CIBMTR). Data analysis.
- A survey of ongoing studies evaluating mesenchymal stem cells in Europe. Part I: manufacturing capacities. Part II: clinical studies, focusing on the use of MSC for GVHD (F Dazzi et al, Immune Tolerance Subcommittee). Data analysis.
- A survey of donor lymphocyte infusions following haplotype-mismatched hematopoietic cell transplantation (N Santoro, Immune Effector Cells subcommittee). Manuscript in preparation
- A survey to define the impact of SARS-COV-2 on delivery of CAR T cell therapy in Europe (S Gorashian, F Malard, Meltem Kurt Yuksel and others; Immune Effector Cells Subcommittee). Data analysis.
- Use of the HLA-B leader to optimize cord-blood transplantation (Annalisa Ruggeri, E Gluckman, Stem Cell Subcommittee; collaboration with the Fred Hutchinson Cancer Research Center, E Petersdorf). Published.
- A survey on cell therapy in solid tumors, launched together with ESMO. (Solid Tumors Subcommittee, P Pedrazzoli) Collecting data.
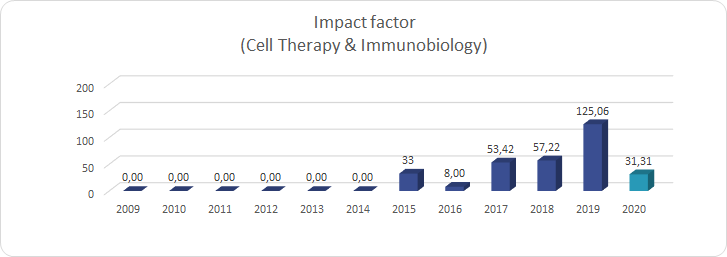
Key publications
| 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | |
|---|---|---|---|---|---|---|
| Oral presentations | 4 | 1 | 7 | 3 | 16 | 8 |
| Poster presentations | 4 | 8 | 3 | 2 | 2 | 1 |
| International educational events | 1 | 1 | 3 | 6 | 6 | 4 |
Major educational courses
- 2nd European CAR T Cell Meeting – Jan 30, 2020 - Feb 1, 2020 / Sitges, Spain.
- Delivery of the Jon van Rood Award to Cristina Toffalori, EBMT 46th Annual Meeting - Aug 29 – Sep 1, 2020 / [Virtual].
- 9th Cell Therapy Day, EBMT 46th Annual Meeting - Aug 29 – Sep 01, 2020 / [Virtual].

