October 2023 Clinical Case of the Month
Title: A New Therapeutic Approach: Fecal Microbiota Transplantation (FMT) For Steroid-Refractory Acute Graft-Versus-Host Disease (SR-aGVHD) Associated with Clostridium Difficile Infection
Submitted by: Lavinia Eugenia Lipan, MD, postdoctoral researcher Hematology, Fundeni Clinical Institute, Bucharest, Romania
Physicians expert perspective: Alina Daniela Tanase, MD, PhD, Professor of Medicine, Immunology of Transplant, Fundeni Clinical Institute - Bone Marrow Transplantation Program, University of Medicine and Pharmacy “Carol Davila”, Bucharest, Romania
A 43-year-old male was diagnosed with acute myeloid leukemia (AML) with CD56 expression, intermediate risk, in May 2022. He received treatment with “7+3” induction regimen with Cytarabine and Idarubicin. He obtained complete remission (CR) with positive minimal residual disease (MRD) (4% with a sensitivity of 10-3 evaluated by flow-cytometry). He continued with high-dose cytarabine-based chemotherapy (HiDAC) for three cycles, remaining in CR with MRD positivity. In this time frame he had multiple infectious episodes for which he received multiple lines of antibiotic and antifungal therapy. He was referred for allogeneic hematopoietic stem cell transplantation (allo-HSCT). In November 2022 he underwent allo-HSCT from a sibling donor. He received myeloablative conditioning regimen FLU/BU4 (fludarabine 150mg/kg and busulfan 12,8mg/kg). The GVHD prophylaxis was based on cyclosporine and MTX. Disease evaluation at day 30 showed no evidence of leukemia by morphology and MRD negativity by flow cytometric evaluation (10-3 sensitivity), with 88% donor chimerism. We started to taper the immunosuppression.
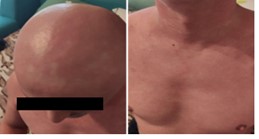
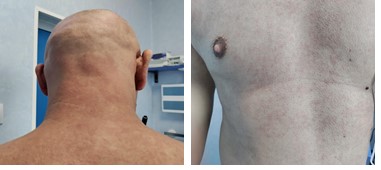
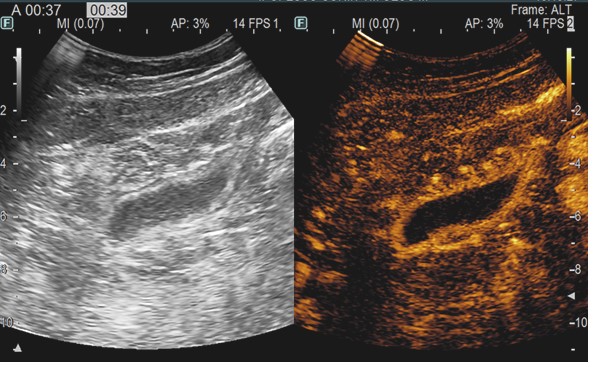
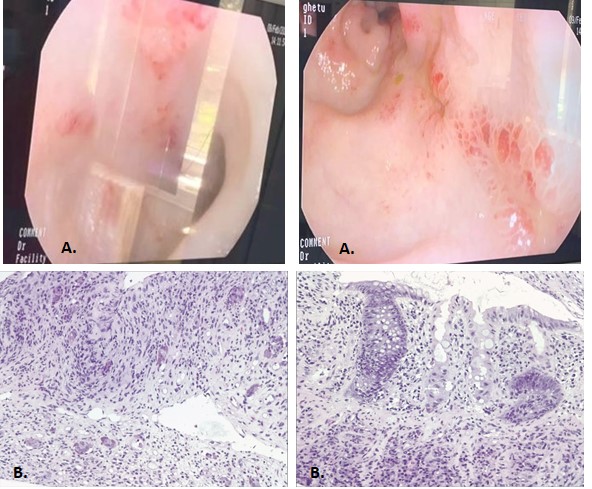
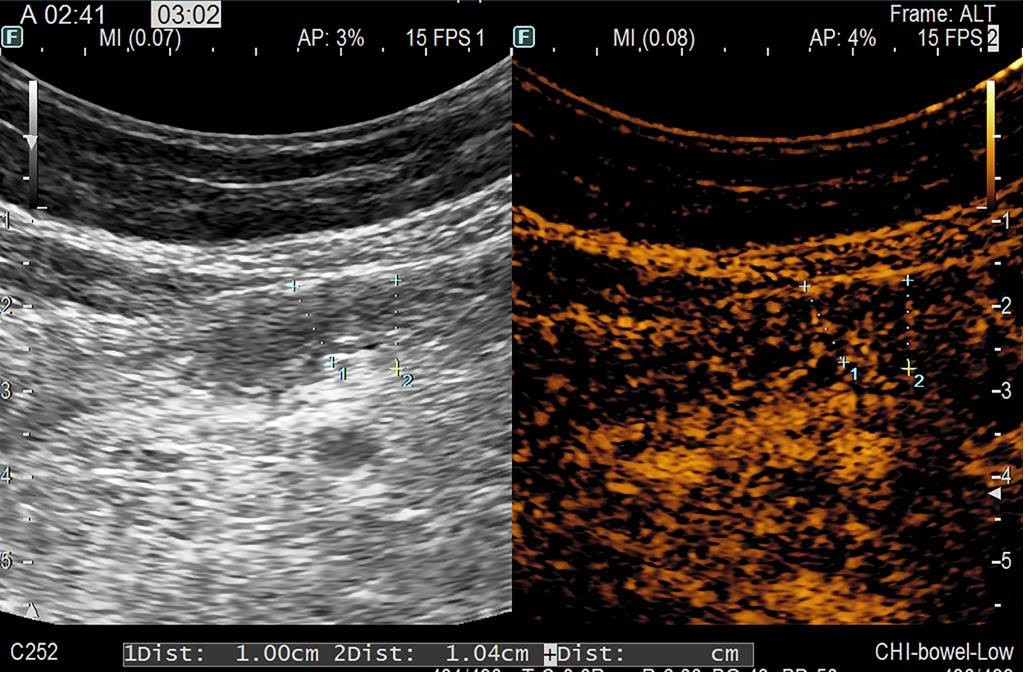
On day 60 post-transplant he was readmitted with stage III cutaneous acute GVHD (aGVHD) (Picture 1). First-line glucocorticoid (GC) therapy was initiated with methylprednisolone 2mg/kg/day. The initial response was favorable at 7 days (Picture 2) and we proceeded to tapering. 2 days later the patient presented with rectorrhagia and watery diarrhea with active Clostridium difficile infection (CDI) (detected by A/B toxins & positive PCR) and oral vancomycin plus i.v. metronidazole was initiated. On contrast-enhanced ultrasonography (CEUS) we discovered important inflammation of the small bowel (Picture 3), and the colonoscopy and histopathological examination (Picture 4) of the gut biopsies established the grade IV aGVHD diagnosis. The patient was considered to have an association between SR-aGVHD and CDI, therefore second-line treatment with ruxolitinib and fidaxomicin was initiated.
After 2 weeks there was no significant improvement and the patient was refractory to the second-line treatment for both conditions.
Which of the following is the most appropriate approach for this patient?
- Escalation of aGVHD treatment (ATG, ECP, Etanercept, etc.)?
- Escalation of CDI treatment (Bezlotoxumab etc)?
- Escalation both of GVHD and CDI treatment per specific center protocol?
- Fecal microbiota transplantation as alternative treatment option for both conditions?
Expert opinion:
Acute GVHD occurs in 30-50% of allo-HSCT patients, out of which 14-36% develop severe forms. Patients with SR-aGVHD have a poor prognosis, with high morbidity and mortality rates, a higher prevalence of opportunistic infections and limited therapeutic options.1 The choice for third line treatment is still a matter of debate and each center’s decision depends on the available therapeutic options. Fecal microbiota transplantation (FMT) has been investigated as a potential treatment for GVHD due to its ability to modulate the gut microbiota, which plays a role in immune regulation. The rationale is that altering the gut microbiota through FMT may help mitigate GVHD symptoms and showed encouraging preliminary results in recent years.2 Yin et. al evaluated the efficacy of ruxolitinib and FMT in the treatment of grade III-IV SR-aGVHD. The ORR on day 28 was 71.4% (95% CI 50.4-92.5%) with higher ORR (76.2%) observed in patients with gut involvement.3 The efficacy of FMT in the treatment of grade IV SR-aGVHD was also evaluated in a non-randomized open-label, phase I/II clinical study, in which clinical remission, event-free survival and overall survival were significantly better for patients undergoing FMT.4 FMT has also proven to be an efficient approach in treating CDI.5,6
Based on the literature review and available therapeutic options, the patient received FMT, after the approval from the ethics committee of our institution. After the 2nd FMT application the patient had a significant clinical improvement, with complete resolution of symptoms after the 4th FMT, without any toxicities. At 8-months post-transplant the patient had complete resolution of symptoms and the CEUS evaluation showed no signs of inflammation of the intestinal wall (Picture 5). Our encouraging finding highlights FMT as a viable and encouraging treatment option with promising results in the treatment of SR-aGVHD associated with CDI. However, there is still a need for large prospective studies to further evaluate these results.
Correct answers: C, D
References:
- Malard F, Huang XJ, Sim JPY. Treatment and unmet needs in steroid-refractory acute graft-versus-host disease. Leukemia. 2020 May;34(5):1229-1240. doi: 10.1038/s41375-020-0804-2. Epub 2020 Apr 3. PMID: 32242050; PMCID: PMC7192843.
- Biliński J, Jasiński M, Basak GW. The Role of Fecal Microbiota Transplantation in the Treatment of Acute Graft-versus-Host Disease. Biomedicines. 2022 Apr 1;10(4):837. doi: 10.3390/biomedicines10040837. PMID: 35453587; PMCID: PMC9027325.
- Liu Y, Zhao Y, Qi J, Ma X, Qi X, Wu D, Xu Y. Fecal microbiota transplantation combined with ruxolitinib as a salvage treatment for intestinal steroid-refractory acute GVHD. Exp Hematol Oncol. 2022 Nov 9;11(1):96. doi: 10.1186/s40164-022-00350-6. PMID: 36352455; PMCID: PMC9644456.
- Zhao Y, Li X, Zhou Y, Gao J, Jiao Y, Zhu B, Wu D, Qi X. Safety and Efficacy of Fecal Microbiota Transplantation for Grade IV Steroid Refractory GI-GvHD Patients: Interim Results From FMT2017002 Trial. Front Immunol. 2021 Jun 17;12:678476. doi: 10.3389/fimmu.2021.678476. PMID: 34220825; PMCID: PMC8248496.
- Liubakka A, Vaughn BP. Clostridium difficile Infection and Fecal Microbiota Transplant. AACN Adv Crit Care. 2016 Jul;27(3):324-337. doi: 10.4037/aacnacc2016703. PMID: 27959316; PMCID: PMC5666691.
- Alonso CD, Maron G, Kamboj M, Carpenter PA, Gurunathan A, Mullane KM, Dubberke ER. American Society for Transplantation and Cellular Therapy Series: #5-Management of Clostridioides difficile Infection in Hematopoietic Cell Transplant Recipients. Transplant Cell Ther. 2022 May;28(5):225-232. doi: 10.1016/j.jtct.2022.02.013. Epub 2022 Feb 22. PMID: 35202891.
Future Clinical Case of the Month
If you have a suggestion for future clinical case to feature, please contact Anna Sureda.