JACIE Activity Report 2023


JACIE celebrated its 25th Anniversary in 2023. Since 2003 JACIE has carried out 878 inspections in 32 countries. Of these, 48% have been initial inspections and 52% are re-accreditations. This has been only possible by the huge commitment and dedication by the Centres, Inspectors and all the Committee members supporting the process.
JACIE’s goal – to harmonise and continually improve quality control standards in cellular therapy, initially across Europe, and now, beyond - remains as relevant as ever for all working in the field of Stem Cell transplantation and Cellular Therapies.
Activity update
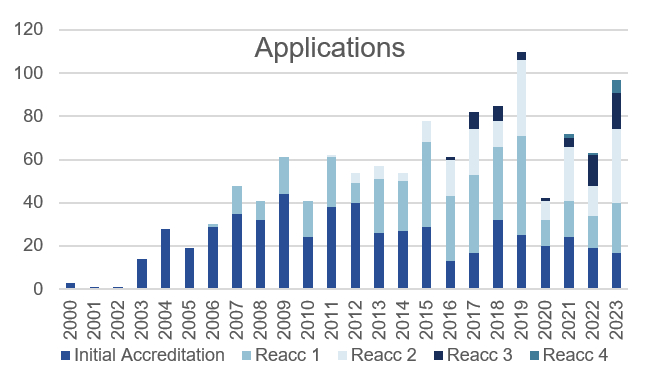
JACIE received 97 applications in 2023 which is approximately 30 % increase in comparison to 2022. 17% of the applications were for the initial accreditation with 6 centres (6%) applying for the 4th re-accreditation.
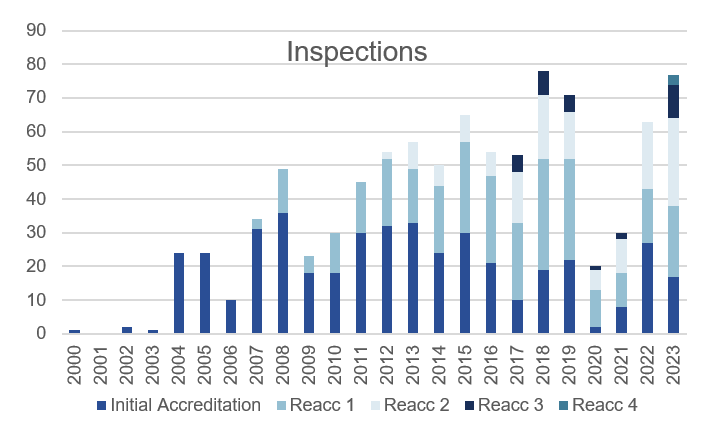
The number of inspections carried out by our inspectors increased to 77, which is the second highest number of inspections in JACIE history (2019: 78 inspections). This shows that JACIE has firmly returned to pre-pandemic activity and we are hugely grateful for all our inspectors, many of whom have carried out 2 or even 3 inspections in the course of the year.
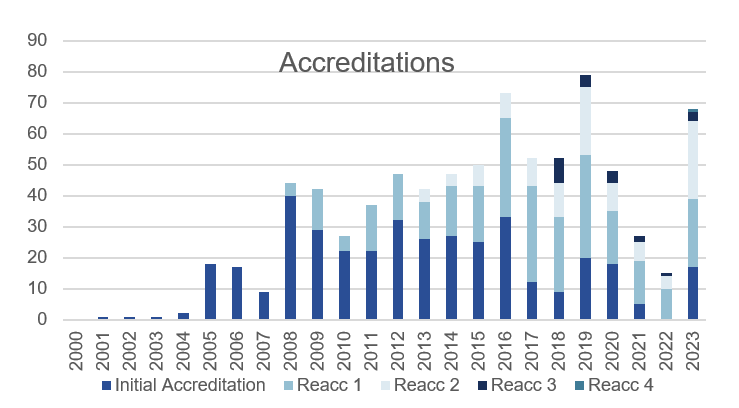
The number of centres accredited was 68 in comparison to 15 in 2022 and 27 in 2021. Huge thank you to the members of the Accreditation Committee who tirelessly reviewed the reports and for the Centres for the timely responses and for the inspectors for assessing the Evidence of Corrections submitted by the Centres. Achieving the JACIE Accreditation is nothing but teamwork.
Institutions accredited in 2023
| Facility | Institution | Country | City |
|---|---|---|---|
| GhdC Charleroi - Autologous Stem Cells Transplantation Program | Grand Hôpital de Charleroi GHdC asbl – Onco-Hematology Department | Belgium | Charleroi |
| Ziekenhuis Netwerk Antwerpen - ZNA | Ziekenhuis Netwerk Antwerpen - ZNA | Belgium | Antwerp |
| Hematopoietic Stem Cell Transplant Program CHU UCL Namur | CHU UCL Namur (site Godinne) | Belgium | Yvoir |
| Hematopoietisch stamceltransplantatieprogramma | AZ Delta | Belgium | Roeselare |
| Stem Cell Transplantation | Centre UZ Gent | Belgium | Gent |
| Stem Cell Transplantation Programme - Hematology | UZ Brussel | Belgium | Brussels |
| Serviço de Transplante de Medula Óssea – Hospital São Rafael | Hospital São Rafael | Brazil | Salvador - Bahia |
| Department of Internal Medicine, Hematology and Oncology | University Hospital Brno | Czech Republic | Brno |
| Helsinki University Central Hospital, HUS Diagnostic Center, Stem Cell Laboratory | Helsinki University Central Hospital, HUS Diagnostic Center, Stem Cell Laboratory | Finland | Helsinki |
| Centre Rouennais de Transplantation Pédiatrique de CSH | Centre Hospitalo-Universitaire de Rouen | France | Rouen |
| Service d’hématologie et de thérapie cellulaire | Hôpital Henri Mondor | France | Créteil |
| Service d’Aphérèse – SHOP (Service d’Hématologie et Oncologie Pédiatrique) & Centre de Biothérapie d’Auvergne (CBA) – CHU Estaing | CHU – site Estaing - Service d’Aphérèse – SHOP & Centre de Biothérapie d’Auvergne | France | Clermont-Ferrand |
| Centre Léon Bérard | Centre Léon Bérard | France | Lyon |
| Transplantation Program, Saint Louis Hospital AP-HP | Hôpital St. Louis Ap-HP | France | Paris |
| Nice Cellular Therapy (NCT) | Hopital Archet 1 – CHU de Nice | France | Nice |
| JACIE-CAEN | CHU Caen Normandie | France | Caen |
| EFS PACA CORSE | Laboratoire de Thérapie Cellulaire – Banque de cellules | France | Saint Laurent du Var |
| Centre Hospitalo-Universitaire de Poitiers /Establissement Français du Sang | CHU de Poitiers | France | Poitiers |
| Pediatric Hematology Oncology - Toulouse | University Hospital of Toulouse – Children’s Hospital | France | Toulouse |
| CHU de LILLE - Centre hospitalo-Universitaire de Lille, Unité de Thérapie Cellulaire and Centre Médico-Chirurgical Ambulatoire (CMCA) |
Hôpital Claude Huriez | France | Lille |
| Hämatopoetische Stammzelltransplantation | Universitätsklinikum Magdeburg AoR | Germany | Magdeburg |
| Klinik für Innere Medizin III | Universitätsklinikum Ulm | Germany | Ulm |
| III. Medizinische Klinik, Universitätsmedizin Mannheim and Institut für Transfusionsmedizin und Immunologie Medizinische Fakultät Mannheim der Universität Heidelberg, DRK-Blutspendedienst Baden-Württemberg – Hessen gGmbH | Germany | Mannheim | |
| Dortmunder Centrum für Zelltransplantation - Standort St.-Johannes-Hospital (DCZ-JoHo) | Dortmunder Centrum für Zelltransplantation - Standort St.-Johannes-Hospital (DCZ-JoHo) | Germany | Dortmund |
| Division for SCT, Immunology and Intensive Medicine, Dept. for Children and Adolescents, University Hospital Frankfurt - Pediatric Stem Cell Transplant Center Frankfurt/Main | JWG - Johann Wolfgang Goethe University - University Hospital (Universitätsklinikum) | Germany | Frankfurt/Main |
| DRK-BSD BaWüHe Frankfurt | DRK-BSD BaWüHe Frankfurt | Germany | Frankfurt/Main |
| Stem Cell Transplantation and Cellular Therapy Unit | Evaggelismos Hospital | Greece | Athens |
| St. Vincent University Hospital | St. Vincent University Hospital | Ireland | Dublin |
| BMT-Processing laboratory | Sheba Medical Center, Tel-Hashomer | Israel | Ramat-Gan |
| Programma Congiunto Trapianto CSE e Terapia Cellulare Azienda Ospedaliero Universitaria Pisana (AOUP) | Azienda Ospedaliero Universitaria Pisana (AOUP) | Italy | Pisa |
| Programma Trapianti | Azienda Ospedaliera di Perugia | Italy | Perugia |
| PROGRAMMA TRAPIANTI | Ospedale A.Perrino | Italy | Brindisi |
| Azienda Ospedaliero-Universitaria Consorzionale | Policlinico di Bari | Italy | Bari |
| Programma Trapianti Ospedale Niguarda | Grande Ospedale Metropolitano Ospedale Niguarda | Italy | Milan |
| Programma Trapianti Cellule Staminali Emopoietiche Sezione Aferesi Terapeutica, Laboratorio di Manipolazione e Criopreservazione Cellulare Servizio Immunotrasfusionale e Genetica Umana, Hematology Department |
Ospedale San Bortolo | Italy | Vicenza |
| Centro Trapianto Midollo Osseo (CTMO) - Clinica Pediatrica dell’Università di Milano - Bicocca Fondazione MBBM | Centro Trapianto Midollo Osseo (CTMO) Clinica Pediatrica dell’Università di Milano- Bicocca Fondazione MBBM | Italy | Monza |
| UOC Ematologia e Trapianto Emopoietico | Ospedale AORN San Giuseppe Moscati | Italy | Avellino |
| Programma Trapianto di CSE in Pediatria | IRCCS Azienda Ospedaliero-Universitaria di Bologna, Policlinico di S. Orsola | Italy | Bologna |
| AOR Villa Sofia-Cervello | AOR Villa Sofia-Cervello | Italy | Palermo |
| Hematopoietic Stem Cell Transplant | American University of Beirut Medical Centre | Lebanon | Beirut |
| Centro de Hematologia y Medicina Interna | Clinica Ruiz | Mexico | Puebla |
| Centre for Cell Therapy (Centrum voor Celtherapie) | The Netherlands Cancer Institute - Antoni van Leeuwenhoek (NKI-AVL) | Netherlands | Amsterdam |
| Laboratory for Cell Therapy | Sanquin Blood Supply Foundation | Netherlands | Amsterdam |
| Haemopoietic Cell Therapy & Transplant Programme, Department of Haematology |
Singapore General Hospital | Singapore | Singapore |
| Blood Transfusion Centre of Slovenia - Department of Therapeutic Services - Collection Centre | Blood Transfusion Centre of Slovenia | Slovenia | Ljubljana |
| Programa de TPH, Department of Hematology | Hospital Universitario Reina Sofía | Spain | Córdoba |
| Hospital Germans Trias i Pujol. | Institut Català d'Oncologia | Spain | Badalona |
| Hematology Department | Hospital de la Santa Creu i Sant Pau | Spain | Barcelona |
| Haemopoietic transplant and cellular therapy programme | University Hospital Virgen del Rocio | Spain | Sevilla |
| Programa de Trasplante y Terapia celular | Hospital Universitario de Salamanca | Spain | Salamanca |
| Unidad de Trasplante | Hospital Universitario de Gran Canaria Dr. Negrín | Spain | Las Palmas de Gran Canaria |
| Centro de Transfusión de la Comunidad Valenciana | Spain | Valencia | |
| BST - Hospital Arnau de Vilanova | Banc de Sang i Teixits | Spain | LLeida |
| Karolinska University Hospital Huddinge | Cellular therapy and allogeneic stem cell transplantation, Clinical Immunology and Transfusion Medicine | Sweden | Stockholm |
| HSCT-programmet, Akademiska Sjukhuset | University Hospital | Sweden | Uppsala |
| Barncancercentrum, The Queen Silvia Children's Hospital, Sahlgrenska University | Barncancercentrum | Sweden | Göteborg |
| Klinik Hirslanden, Medizinisches Programm für Zelltherapie | Klinik Hirslanden, Medizinisches Programm für Zelltherapie | Switzerland | Zürich |
| Southeast Scotland Haematopoietic Stem Cell Transplant Service | Western General Hospital, Scottish National Blood Transfusion Service (SNBTS) | United Kingdom | Edinburgh |
| Stem Cell and Immunotherpay Department (SCI) Therapeutic Apheresis Service (TAS) | NHS Blood and Transplant | United Kingdom | Oxford |
| Belfast City Hospital | Belfast Health and Social Care Trust | United Kingdom | Belfast |
| Haematopoietic Stem Cell Transplant Programme | Barts Health NHS Trust - St Bartholomew's Hospital | United Kingdom | London |
| St George’s University Hospital | St. George's University Hospital - NHS Trust | United Kingdom | London |
| The London Clinic | The London Clinic | United Kingdom | London |
| Paediatric Haematopoietic Stem Cell Transplantation & Bone Marrow Collection Programme - Royal Hospital for Children | Royal Hospital for Children (NHS GREATER GLASGOW AND CLYDE) | United Kingdom | Glasgow |
| Nottingham University Hospital NHS trust | Nottingham University Hospital City Campus | United Kingdom | Nottingham |
JACIE Inspectors
The JACIE team continued the inspector training with the new format following the launch in November 2023. Three Inspector Training Courses took place in Barcelona, Paris and Istanbul with a total of 55 new inspectors trained. The Paris and Istanbul courses were run in collaboration with the National Transplant Societies and on behalf of JACIE we would like to extend our heartfelt thanks to these Societies for their support and collaborations.
JACIE launched the JACIE Inspector Handbook at the Annual Meeting in April 2023. The Handbook is provided to all inspectors to support them in their role with advice on the processes whether on how to book the travel or claim expenses to challenging Standards and how to assess them. The Handbook was developed in collaboration with the JACIE Inspector Committee who worked with the JACIE team to ensure that the information was both relevant and up to date.
Inspector Committee members also developed a proposal for improved Inspector Incentives, which was submitted for approval by the EBMT Board. This proposal was based on the survey in July 2023 that was sent to all active inspectors. Further updates will be available in 2024. However, as one of the first steps, JACIE was able to offer 72 grants for JACIE Inspectors to attend the EBMT 50th Annual Meeting in Glasgow 2024.
The Inspector Committee continued with the Webinar series for the Inspectors sharing best practice and updates on process and Standards.
Accreditation e-course for Centres
Together with the QMC, JACIE created the JACIE Accreditation e-course for Centres, a new, online, easy access, accreditation preparation course which aims to help all staff working in centres feel more confident in preparing for and undergoing the JACIE Inspection. The modules present information in a variety of formats, including presentations from experienced JACIE Quality Management Committee members as well as JACIE Accreditation Coordinators. The key objectives of the course is to help the Centre staff to understand the application process, to improve the understanding of some of the challenging Standards and how to comply, and lastly, to reduce any stress/anxiety around the accreditation process by supporting the Centres throughout the process.
This new e-course is available to both members and non-members of the EBMT and is free to access at any time.
9th Edition Standards review process
FACT-JACIE Standards are reviewed every three years and the latest process for the 9th Edition of Standards commenced in June 2023 with a Steering Committee ‘kick-off’ meeting in June. The Steering Committee manages the overall process as well as makes all strategic decisions on the Standards.
Five sub-committees (Clinical, Collection, Processing, Quality Management, and IEC) commenced their work in September 2023 with the work coordinated by the FACT office. The work will continue in 2024 with the Steering Committee meeting planned for February 2024, public consultation for summer 2023 and finally publication of the 9th edition in March 2025.