The EBMT Registry 2022

The new EBMT Registry is advancing, slowly but with determination. I am glad to announce that today we are very confident that the New Registry will go live during 2023. Although we have had challenges and delays, also big progress has been achieved due to our dedicated team.
Since 2022, the EBMT together with National Registries have worked to develop the new tool that will take ProMISe's place: the EBMT Registry, its first version will be with some limitation in its functionalities.
Together with the EBMT Working Parties, we have revised the contents within our data collection forms. We have restructured the way we will collect data. The new data collection forms will be split up into patient registration, diagnosis, treatment, complications and follow-up, which allows for mixing and matching based on needs. These forms will be the core dataset of the new database.
The mapping of ProMISe data has started so it can be migrated to the new platform. At the time of launch, there will be only partly migrated patient data from ProMISe to the new EBMT Registry. The rest of the data will be migrated as soon as possible.
Furthermore, we have adopted a new data model, called OMOP-CDM, which will facilitate in the future fruitful collaboration with other Registries, a new, standardised European scientific way of presenting the data ready for all types of analysis.
The data collection forms and manuals are essential in order to prepare the training on the new core dataset. The training strategy will be presented during the 49th Annual Meeting of the EBMT in Paris.
The collaboration with EBMT and National Registries has been key in this designing phase. Regular monthly meetings were organised with National Registries and 9 individual meetings between representatives of each national society.
We would also take this opportunity to share with you the good news about an EU grant awarded to EBMT and 10 other entities. It has been a long process that started when the proposal was submitted in January 2022, and it has finally been approved this month. The EU grant guarantees financial stability to the EBMT Registry project and enhances EBMT's networking.
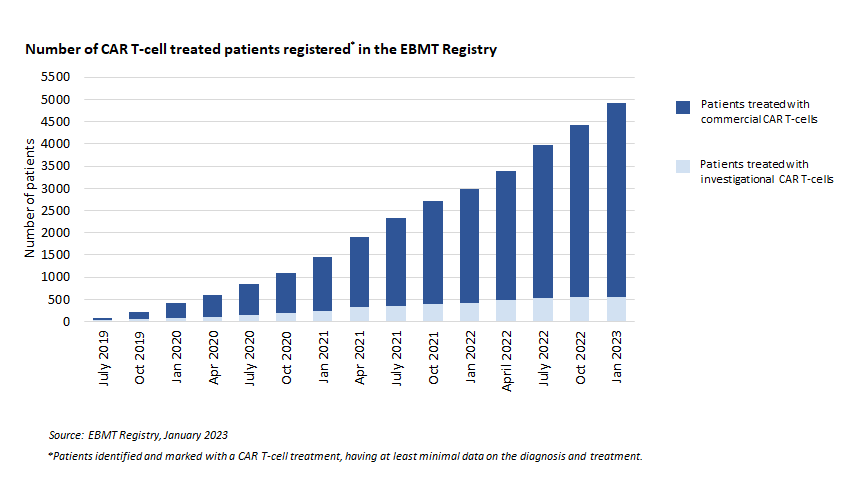
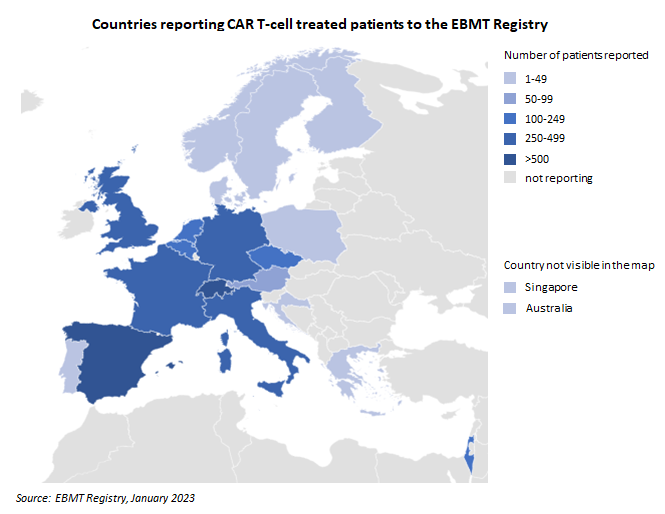
The Castor platform was launched with the collection of 4,600 CAR T cell patients. The handbook for data entry has been updated recently. Specifically, the terminology has been amended to match the platform and information on the registration of second treatments at Castor has been added.
Registrations for Cellular Therapy