JACIE Activity Report 2020

Standards and Accreditation – JACIE
The Joint Accreditation Committee ISCT-Europe & EBMT (JACIE) was established in 1998. It promotes high-quality patient care and laboratory performance in the collection, processing and administration of cellular therapy through a profession-led, voluntary accreditation scheme.
JACIE works continuously with international partner organisations to develop and maintain standards for the provision of quality medical and laboratory practice in HSCT, performs on-site inspections, and accredits those programmes that demonstrate compliance with these standards. JACIE also provides training for inspectors and centres on the accreditation process.
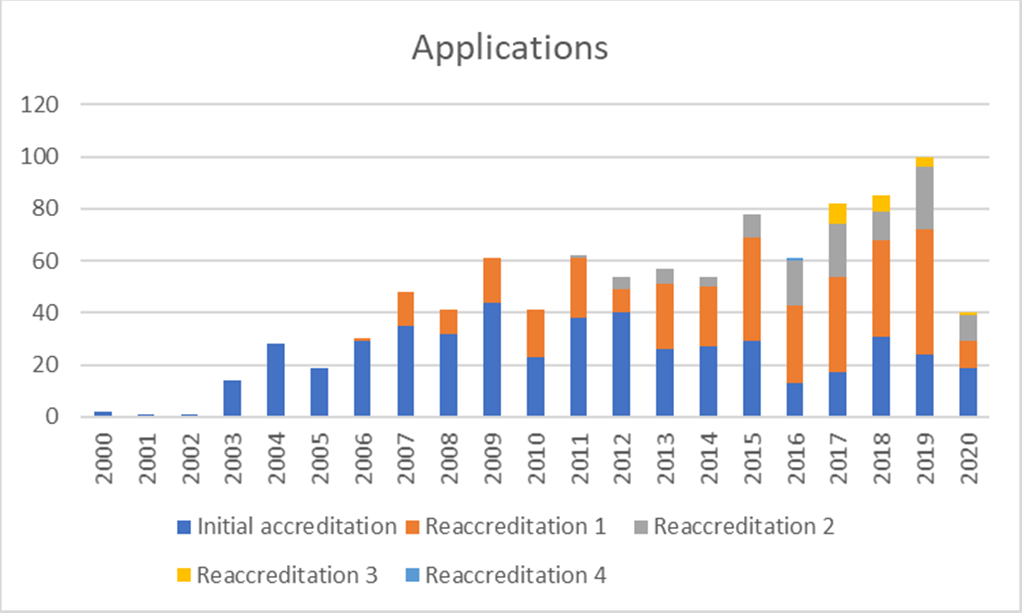
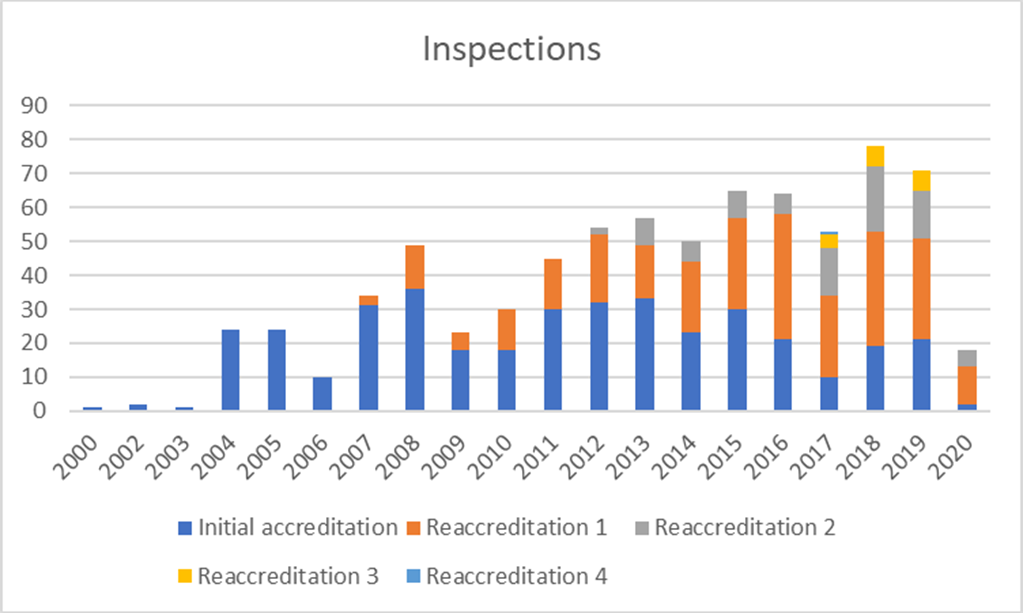
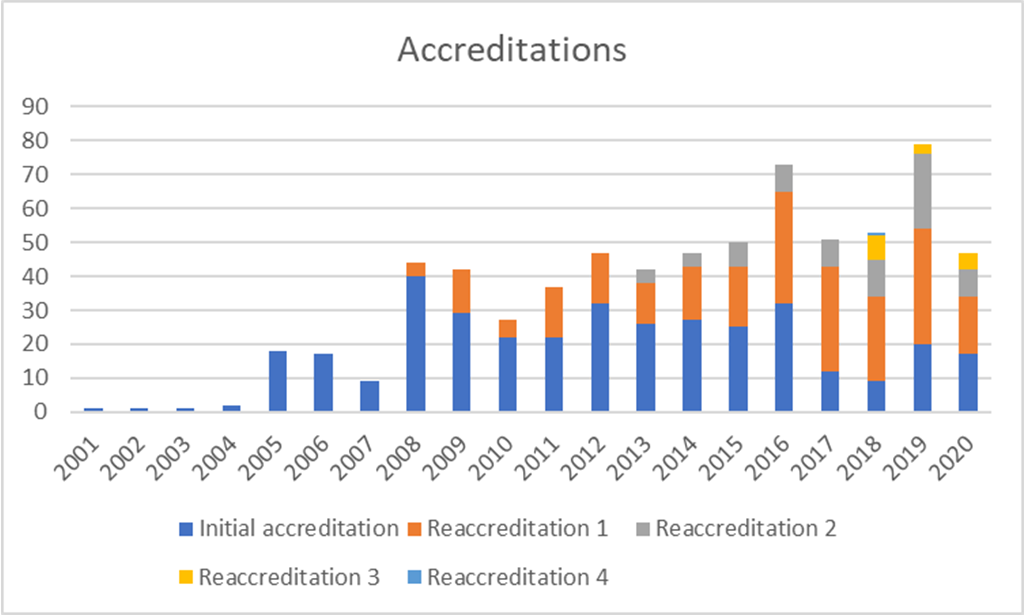
Since 2000, 492 transplant programmes and facilities in 34 countries in Europe and beyond have applied to JACIE and 753 inspections (first-time and reaccreditation) have been performed. 362 applicants have achieved accreditation at least once with practically all centres repeating the process after the first accreditation cycle. There are over 300 registered inspectors, all volunteers drawn from the HSCT and cellular therapy field.
Following the first two joint JACIE-FACT inspections performed in Latin America in 2018, further centres from the region have presented themselves to be inspected during 2021. This ‘step-wise’ format is being piloted in collaboration with the Latin American Group for Bone Marrow Transplantation (LABMT).
In terms of activity, 2020 was severely affected by the pandemic from mid-March. 40 applications were received, 18 inspections were completed before on-site inspections were cancelled and 47 accreditations were awarded.
JACIE is developing a remote inspection process to be launched in the first quarter of 2021.
Applications
Inspections
Accreditations
Benchmarking
The FACT-JACIE Standards require centres to review their clinical outcomes against wider datasets, referred to as benchmarking. EBMT as a registry holder is in a unique position to offer an international benchmarking scheme to centres that report data. In October 2020, the first phase of this service was launched whereby reports of data-completeness were provided to 416 qualifying centres for the period 2013-2016. The second phase in February 2021 will see the provision of clinical outcome benchmarking reports to those centres that have met the data completeness threshold.
Benchmarking will thereafter become an annual exercise. The next edition will be in June 2021 based on 2015-2019 data. Full information on the scheme can be found in the Snowden et al, 2020 paper cited below.
8th Edition of FACT-JACIE Standards
The process to prepare the 8th edition of the FACT-JACIE Standards continued throughout 2020. The first draft was opened for public consultation in April 2020 for 3 months. 770 comments from 78 participants in 12 countries were received and that feedback was reviewed by the Standards sub-committees during the last quarter of 2020 before the final draft will be presented to the FACT and EBMT Boards for approval in early 2021.
The definitive text will be published in May 2021.
Governance
JACIE Committee
Dr. Kim Orchard (Southampton, UK) succeeded Prof. John Snowden as Chair of the JACIE Committee in mid-2020. Prof. Snowden, now the EBMT Secretary, remains on the Committee. See https://www.ebmt.org/jacie-committee for more details.
Dr. Isabel Sánchez Ortega joined EBMT in March 2020 as Medical Officer. Among her responsibilities is supporting JACIE. Dr. Sánchez Ortega took over the JACIE Medical Director duties from Dr. Riccardo Saccardi, who occupied this role from January 2016 to June 2020.
Accreditation Committee
Dr. Lynn Manson (Edinburgh, UK) succeeded Dr. Kim Orchard as Chair of the Accreditation Committee in October 2020. See https://www.ebmt.org/accreditation-committee for more details.
Inspectors Committee
The Inspectors Committee was newly formed in October 2020. Members are drawn from the pool of JACIE Inspectors and will have an important influence over the inspection process and related aspects. The Committee is chaired by Ms. Tuula Rintala (London, UK). See https://www.ebmt.org/inspectors/inspector-committee for more details.
Quality Managers Committee
The Quality Managers Committee was established in 2011. All of the members are experienced quality managers or responsible for quality management working in accredited centres or towards accreditation. The Committee is chaired by Ms. Renza Monteleone (Reggio Calabria, IT). See https://www.ebmt.org/quality-managers-committee for more details.
Other
The JACIE Twitter account @JACIE_EBMT has grown to 1,598 followers.
We would like to express our continued appreciation and admiration for the Inspectors, JACIE Committee Members, Accreditation, Standards and Quality Manager Committee members, other volunteers and the JACIE Office team for all their tremendous hard work, commitment and dedication.
Institutions awarded accreditation in 2020
- CHU ESTAING – Service de Thérapie Cellulaire et d’Hématologie Clinique Adulte, Clermont Ferrand, France
- CHU Dijon - Hématologie Clinique, Dijon, France
- Hôpital Robert-Debré, Paris, France
- CHU de Rennes, Rennes, France
- Institut Universitaire du Cancer de Toulouse-Oncopole (IUCT-O), Toulouse, France
- Zentrale Dienstleistungseinrichtung für Transfusionsmedizin/Blutspendezentrale, Uniklinik Köln AöR, Cologne, Germany
- University Hospital Frankfurt - Goethe University, Frankfurt am Main, Germany
- Universitätsmedizin Göttingen, Göttingen, Germany
- Universitätsklinikum Heidelberg, Heidelberg, Germany
- University Hospital Jena, Jena, Germany
- Universitätsklinikum Leipzig AoeR, Leipzig, Germany
- Klinikum rechts der Isar der TU München, München, Germany
- Universitätsklinikum Münster, Klinik für Kinder- und Jugendmedizin Pädiatrische Hämatologie und Onkologie, Münster, Germany
- Universitätsklinikum Münster, Medizinische Klinik A / KMT-Zentrum, Münster, Germany
- Medizinische Klinik 5, Klinikum Nürnberg Nord, Nürnberg, Germany
- Medical Center II, University Hospital Tuebingen (Including Autologous transplantation in Adult patients in Rems-Murr-Klinikum, Winnenden), Tübingen, Germany
- DE KK Institute of Internal Medicine, Debrecen, Hungary
- St. James´s Hospital, Dublin, Ireland
- Sheba MC, Ramat-Gan, Israel
- Sheba MC, Ramat-Gan, Israel
- CRO IRCSS Aviano, Aviano, Italy
- ASST Spedali Civili di Brescia, Brescia, Italy
- Azienda Ospedaliera Santa Croce e Carle, Cuneo, Italy
- Azienda Ospedaliero Universitaria di Modena, Modena, Italy
- Azienda Ospedaliera di Padova, Padova, Italy
- AUSL Romagna-IRST IRCC, Ravenna, Italy
- Institute for Maternal and Child Health - IRCCS Burlo Garofolo, Trieste, Italy
- AUMC, location Academic Medical Centre, Amsterdam, Netherlands
- Radboud University Medical Centre Nijmegen, Nijmegen, Netherlands
- Isala, Zwolle, Netherlands
- King Faisal Specialist Hospital & Research Centre, Jeddah, Saudi Arabia
- Blood Transfusion Centre of Slovenia, Ljubljana, Slovenia
- Hospital Universitario 12 de Octubre, Madrid, Spain
- Hospital Clínico Universitario Virgen de la Arrixaca, Murcia, Spain
- Hospital Clínico de Santiago de Compostela, Santiago de Compostela, Spain
- Sahlgrenska University Hospital, Göteborg, Sweden
- Luzerner Kantonsspital, Luzern, Switzerland
- Kantonsspital St. Gallen, St. Gallen, Switzerland
- NHS Blood and Transplant, Bristol, United Kingdom
- Cambridge University Hospitals NHS Foundation Trust, Cambridge, United Kingdom
- NHS Greater Glasgow & Clyde, Glasgow, United Kingdom
- Leeds Teaching Hospitals NHS Trust, Mid Yorkshire NHS Trust, NHS Blood and Transplant Leeds, Leeds, United Kingdom
- University Hospitals of Leicester (UHL) NHS Trust & Northampton General Hospital NHS Trust (NGH), Leicester, United Kingdom
- University College London Hospitals NHS Foundation Trust, London, United Kingdom
- Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle upon tyne, United Kingdom
- University Hospital Southampton NHS Foundation Trust, Southampton, United Kingdom
- Somerset NHS Foundation Trust, Taunton, United Kingdom
Full list available at www.ebmt.org/jacie-accredited-centres
