Reporting is learning. By reporting data on patients treated with CAR T-cell therapies, you contribute to academic research and regulatory decision making by health authorities. Ultimately, this supports EBMT’s mission to advance cellular and stem-cell based therapies and improve the lives of patients with blood-related disorders.
Data quality is fundamental to doing meaningful analyses. To ensure that we have complete and accurate data, EBMT invites centres that treat patients with commercial CAR T-cell therapies to participate in a Data Collection Initiative to support Post Authorisation Safety (PAS) studies mandated by the European Medicines Agency (EMA). Centres that participate will be financially compensated.
How is the Data Collection Initiative set up to support PAS studies?
The data collection for PAS studies is based on the standard Registry forms and procedures, but a wider infrastructure is being set-up to support PAS studies and reimburse centres for their work.
Background information
With the approval of the first CAR-T therapies, the EMA required MAHs to monitor their products in the long-term. The cellular therapy module of the EBMT Registry received a positive qualification opinion from the EMA, supporting the use of Real-World Data captured in the EBMT Registry to inform regulatory decision making by health authorities.
Frequently Asked Questions (FAQs)
What are the requirements for participation in the Data Collection Initiative?
- To participate in the Data Collection Initiative on CAR-T therapies, a centre must, first and foremost, report data on all patients treated with (commercial) CAR-T therapies in the EBMT Registry as per the standard data collection forms and processes (the Cellular Therapy form). This does not require any additional contracts and/or regulatory approvals. Consequently, this data collection is already ongoing and should continue.
- Secondly, if needed, help the EBMT Clinical Study Unit with obtaining regulatory approval for the updated informed consent form which allows patients to also consent to share the data collected in the Registry with EBMT’s collaboration partners including MAHs and health authorities.
- Thirdly, (re-)consent patients with the updated informed consent form.
- Finally, help to set up contracts between your centre and EBMT which allows EBMT to reimburse your site for the data management efforts. (See the question “What contracts should my centre sign?” below).
What does my centre have to do when participating in the Data Collection Initiative?
- You will need to complete the standard patient's data in the EBMT Registry. In addition, we ask you to comply with data requests (queries) to ensure completeness and quality of the data.
- Data on patients treated with CAR-T therapies needs to be reported at the following time points: baseline, 100 days, 6 months and then yearly up until 15 years (or until death).
- Also, we ask you to consent patients with the updated patient informed consent form, which asks patients to indicate whether they allow sharing of their data with EBMT collaboration partners and source data verification of their data.
- Patients who explicitly consent to share their data with collaboration partners will be included in the dataset that is shared with these collaboration partners. The centre should also be available for on-site monitoring visits.
Why should my centre participate? What is in it for me?
- Participation in the Data Collection Initiative is important because ‘reporting is learning’. The PAS studies are mandated by EMA because we lack information on the long-term safety and effectiveness profiles of CAR-T therapies. As a prominent European medical community, we want to be in the lead of evidence generation of these promising treatments. Collectively we can generate robust real-world evidence to support regulatory decision making as well as scientific studies.
- The current protocols for the PAS studies are based on secondary use of Registry data. With this approach we prevent the creation of additional databases owned by marketing authorisation holders. The EBMT Registry makes data available to the scientific community and it avoids the need for centres to report data to multiple registries. By using a harmonised data collection form for cellular therapies, it will be easier to enter and compare data on different products.
- Centres will receive a generous reimbursement for their data management efforts. This money will be given for all patients treated with commercial CAR-T therapies who (re-)consent to sharing their pseudonymised data with MAHs as per the Product-Specific Agreement between the participating centre and EBMT.
- If you have a good study proposal for a CAR-T-related study, you can submit it to the relevant EBMT Working Parties or the GoCART Coalition. When your proposal is selected/accepted, you will not only have access to the data from your centre but (indirectly) also to data from other centres in your country or the Registry as a whole. Working Parties have dedicated statisticians, data managers and study coordinators to support the conduct of the selected proposals.
What contracts should my centre sign?
- The contracts for the Data Collection Initiative for the PAS studies are split in two parts; 1) the Data Collection Master Agreement and 2) the Product-Specific Agreement. The Data Collection Master Agreement governs the general data collection requirements that are identical for all CAR-T therapies. Whereas the shorter Product-Specific Agreement specifies additional requirements for the individual CAR-T therapies. For each new CAR-T product you will only need to sign a new Product-Specific Agreement.
- By using this set-up, in which the Product-Specific Agreements are incorporated in the Data Collection Master agreement, we aim to reduce the time needed for contract negotiations and thus reduce the start-up timelines and burden for new cellular therapy products included in the Data Collection Initiative.
- We also remind your centre to sign the Joint Controllership Agreement (JCA). This document governs all data processing activities between EBMT and the centre and is thus a prerequisite but not specific to participate in the CAR-T Data Collection Initiative. The JCA should be signed by all centres, including those who do not participate in the CAR-T Data Collection Initiative.
Will my centre be reimbursed?
- If your centre has agreed to participate in the Data Collection Initiative and has signed the relevant contracts, your centre will be reimbursed for all patients treated with commercial CAR-T therapy products that (re-)consent to allow sharing of their data with the marketing authorisation holders and health authorities as per the stipulations in the Product-Specific Agreement. You will only be reimbursed for patients treated after the marketing approval date in the post-marketing setting and when good quality data is entered and can be shared with the MAHs. The specific details of the inclusion period and payment schedule is provided in each Product-Specific Agreement.
- In addition to the Agreements, EBMT will inform the sites which indications are contracted with the pharma company to get paid for those patients. When a new indication is contracted with the pharma company EBMT will inform the sites accordingly. To check the latest information, please check the Study update section.
Find your country. Find your contact.
EBMT has appointed both lead clinical study coordinators and lead principal investigators for each country. Please refer to the list below to identify the contact person in your country.
| Country | Lead EBMT | Country lead PI |
|---|---|---|
| Austria | Guus Rijs | Philipp Wohlfarth, MD, PhD; Medical University of Vienna, Vienna |
| Belgium | Guus Rijs | Sébastien Anguille, MD, PhD; Antwerp University Hospital/University of Antwerp, Antwerp |
| Croatia | Paul Bosman | Prof. Igor Aurer - University Hospital Center Rebro |
| Czech Republic | Paul Bosman | Frantisek Folber, MD, PhD - Department of Internal Medicine, Hematology and Oncology, University Hospital Brno |
| Denmark | Guus Rijs | Soeren Lykke Petersen, Consultant, MD, D. Msc. - Copenhagen University Hospital (Rigshospitalet) |
| Estonia | Jessica Lemaitre | |
| Finland | Dilyana Georgieva | Dr. Mervi Taskinen MD, PhD - University of Helsinki, Helsinki |
| France | Jessica Lemaitre | Pr Stéphanie Nguyen -Universite Paris IV, Hopital la Pitié-Salpêtrière, Paris |
| Germany | George Haroon | Prof. Dr. med. Nicolaus Kröger - University Hospital Eppendorf, Hamburg |
| Greece | Dilyana Georgieva | |
| Israel | Paul Bosman | Prof. Avichai Shimoni - Chaim Sheba Medical Center |
| Italy | Paul Bosman | Dr. Annalisa Ruggeri MD, PhD - Ospedale San Raffaele s.r.l |
| Netherlands | Guus Rijs | Dr. Pim Mutsaers van het Erasmus Medical Center, Rotterdam |
| Norway | Dilyana Georgieva | Tobias Gedde-Dahl d.y. MD PhD - Oslo University Hospital Rikshospitalet, Oslo |
| Poland | Paul Bosman | Prof. Krzysztof Kalwak - Cape of Hope, Wroclaw |
| Portugal | Jessica Lemaitre | |
| Slovenia | Paul Bosman | Dr. Matjaz Sever MD, PhD - University Medical Center Ljubljana |
| Spain | Paul Bosman | Dr. Anna Sureda MD, PhD- ICO- Hospital Duran i Reynals, Barcelona |
| Sweden | Dilyana Georgieva | Prof. Dr. Stephan Mielke - Karolinska University Hospital, Stockholm |
| Switzerland | Jessica Lemaitre | Prof. Dr. med. Caroline Arber Barth - université de Lausanne, Centre hospitalier universitaire vaudois, Lausanne |
| United Kingdom | Dilyana Georgieva | Dr. Sara Ghorashian, Great Ormond Street Hospital, London |
Contact us if your country is not listed at clinicalstudyunit@ebmt.org
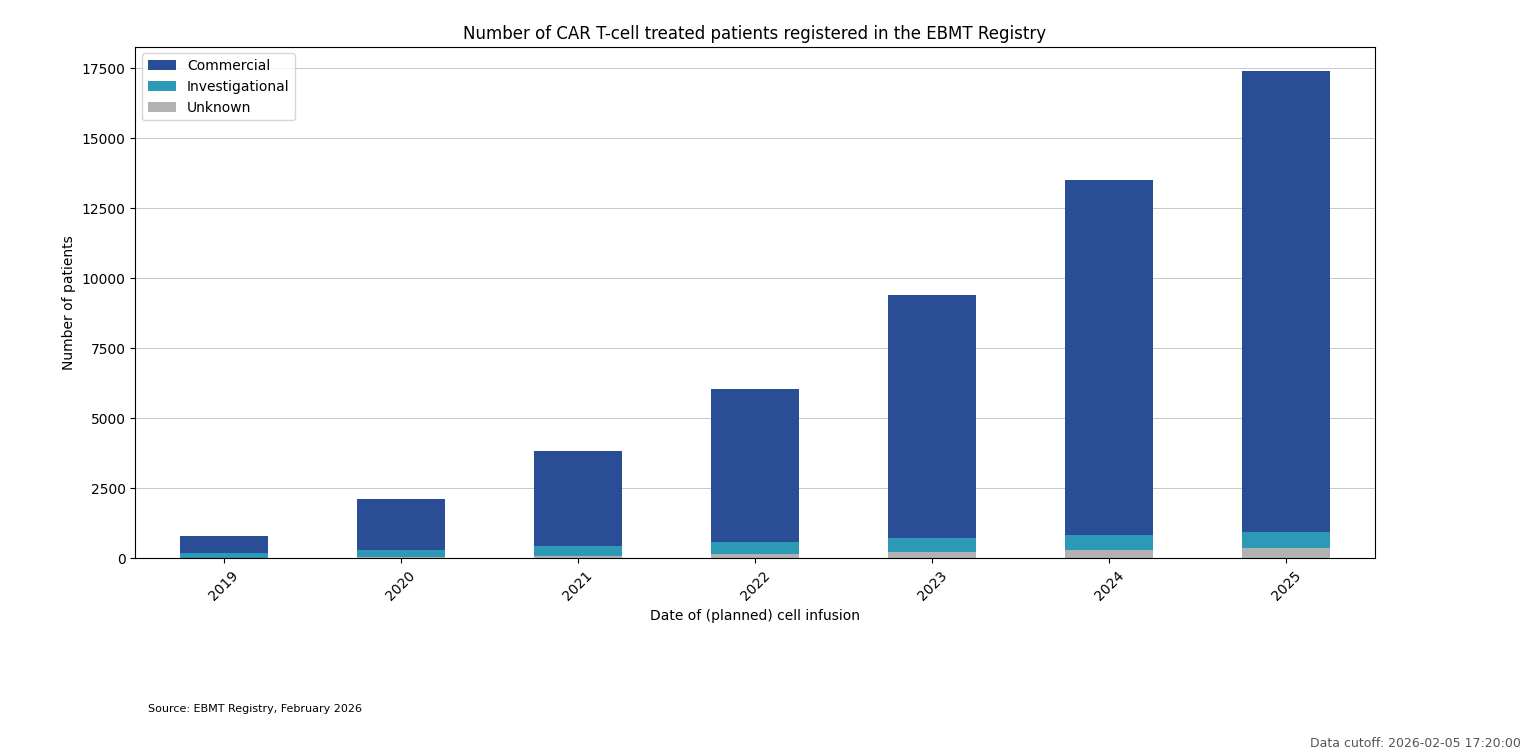
CAR T-cell treated patients registered in the EBMT Registry
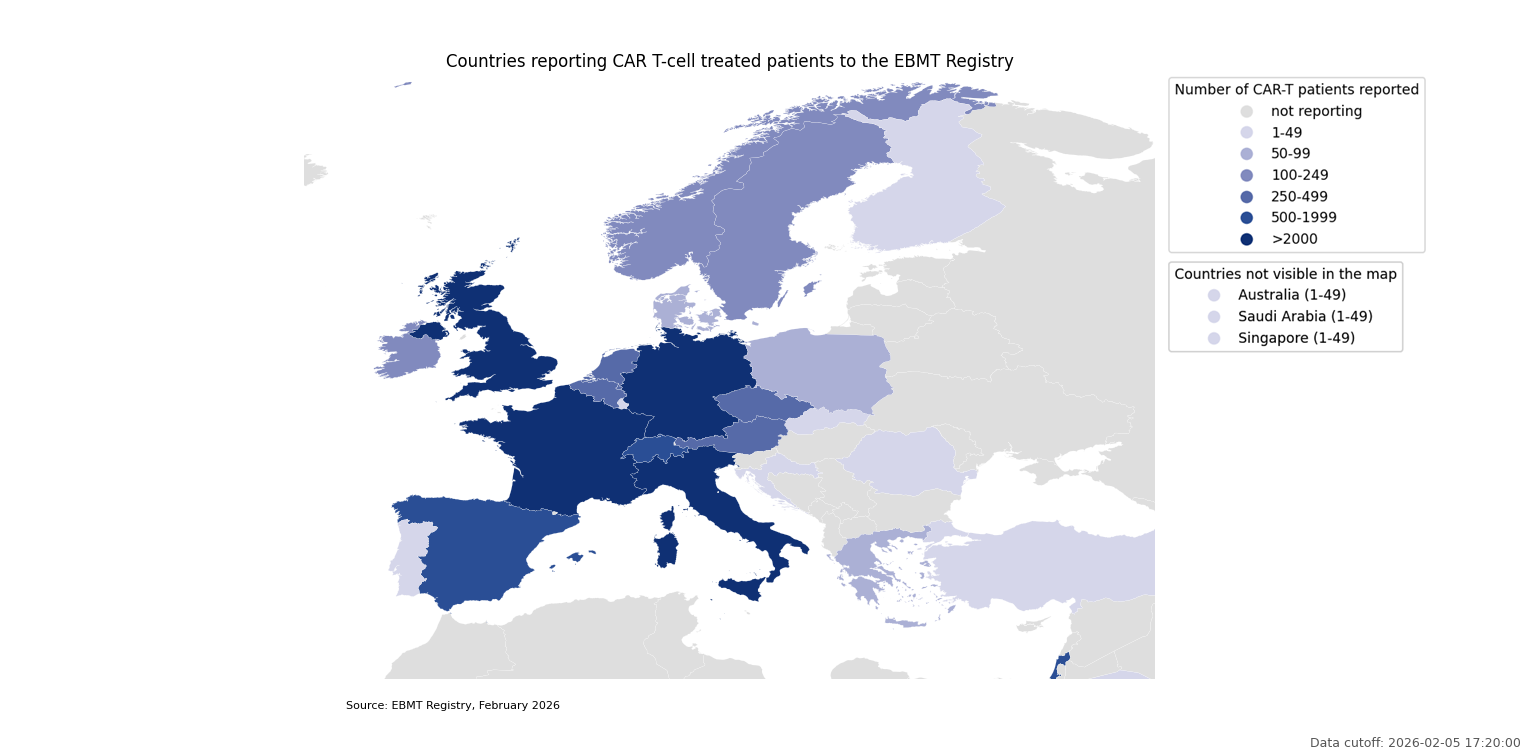
Countries reporting CAR T-cell treated patients to the EBMT Registry
EBMT is a Non-Profit Organisation
EBMT is a non-profit organization supporting the transplant and cellular therapy community. In line with our Mission, Vision and Values, our resources are used to improve the lives of patients with blood-related disorders.
EBMT has created a level of financial reserves to be able to guarantee its independence and neutrality regarding all its scientific and educational decisions from any political and economic interest. All income to EBMT is re-invested to support our community with it scientific, registry, accreditation and educational activities.
Recent activities and outputs in CAR T include: International Training Course, GoCART Consortium, EBMT-JACIE/EHA CAR T Guidelines, EBMT-EHA CAR T Handbook and the CAR T Data Registry amongst others.
For more information about EBMT's involvement in the PAS Studies please visit the FAQs page.
