JACIE Office has recently received number of queries on the minimum activity required for facilities to achieve accreditation. Further, with the changes in clinical practice, meeting the minimum activity required can be challenging for bone marrow collection facilities in particular. Although this can be frustrating for the facilities affected, it is important to remember that these Standards are intended to protect the donors (autologous and allogeneic) and the product quality. The minimum activity requirements for each facility are summarised below.
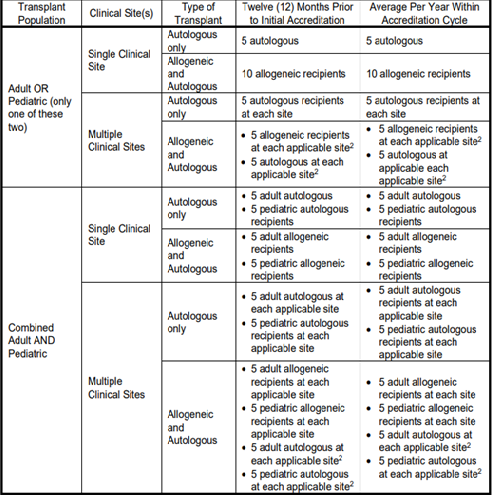
Minimum Activity for Clinical Programmes
Collection (HPC-M) Facility (Standard CM1.5)
A minimum of one (1) marrow collection procedure shall have been performed in the twelve (12) month period immediately preceding initial accreditation, and a minimum average of one (1) marrow collection procedure per year shall be performed within each accreditation cycle.
Collection (HPC-A) Facility (Standard C1.5)
Apheresis facility shall collect a minimum of ten (10) cellular therapy products in the twelve (12) month period immediately preceding initial accreditation, and a minimum average of ten (10) cellular therapy products shall have been collected by apheresis per year within each accreditation cycle. This standard refers specifically to the number of collection procedures for cellular therapy products, not the number of patients from whom cells were collected, and may include both allogeneic and autologous donors.
Processing Facility
There is no minimum activity defined for the Processing Facility. However, as per explanation for Standard D1.3 the Standard requires that during a 12-month period, Processing Facilities need to process enough cellular therapy products to compile an adequate amount of data to validate processes and demonstrate compliance with the Standards. Facilities should have provided service for the minimal number of autologous and allogeneic transplants required for the associated Clinical Program to be eligible for FACT or JACIE accreditation.
Although the Standards do allow Facilities to apply for accreditation prior to the meeting the minimum volume, this is intended for exceptional circumstances only and the Accreditation will not be awarded until the minimum volume is met. Therefore, it is for the Clinical Program or Collection/Processing Facility to decide, if it is in a position to accept the risk of not meeting the minimum volume (and not becoming accredited) within the accreditation timeline.
JACIE office checks the activity of the applicant Centre/Facility at the application and will provide advise at that time on whether or not they meet the minimum activity. If you have any queries regarding the minimum activity requirements prior to applying for initial/re-accreditation, please do not hesitate to contact us on jacie@ebmt.org.