
Title: Progressive Multifocal Leukoencephalopathy After CAR T-Cell Therapy: A Case Responsive to Nivolumab
Submitted by Dr Maxime Delforge
Physicians expert perspectives: Dr Nicolas Gazeau* and Dr David Beauvais
* MD, Hematologist, CHU Lille, Lille, France
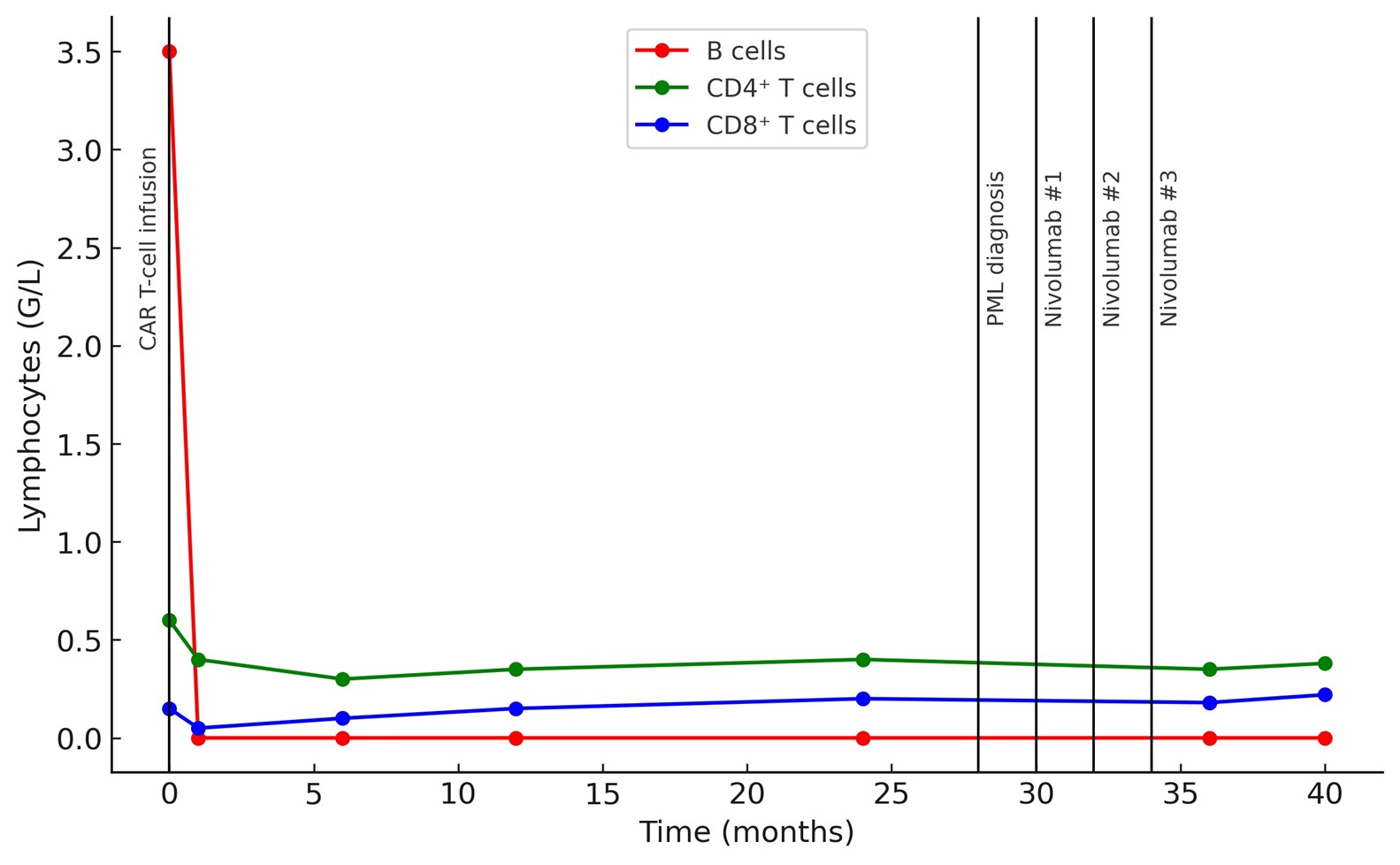
An 82-year-old woman with grade 3A, stage IV follicular lymphoma was treated with CD19 directed CAR T-cells (lisocabtagene maraleucel) after three prior lines of therapy. Baseline brain MRI was normal. The early post-infusion course was marked by grade 1 ICANS on day +6 and by the persistance of complete B-cell aplasia (0 × 10⁹/L) from day +7 and during all the follow-up, consistent with the expected on-target/off-tumor effect of CD19 directed CAR T-cells
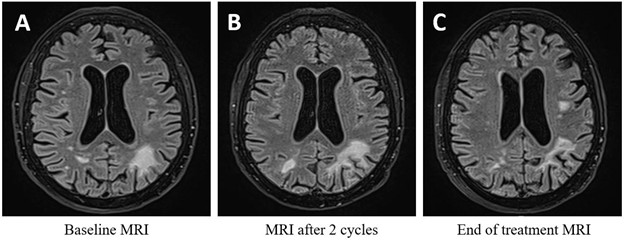
About 30 months after CAR T-cell therapy, she progressively developed cognitive symptoms, including dyslexia, dysgraphia, aphasia, and memory impairment. Brain MRI revealed asymmetric bilateral parieto-occipital T2-FLAIR hyperintensities.
JC virus PCR on cerebrospinal fluid was positive at 12,844 IU/mL (4.11 log₁₀), confirming the diagnosis of progressive multifocal leukoencephalopathy.
At diagnosis, CAR T-cells were still detectable by qPCR (470 copies/10⁶ leukocytes; 2.67 log₁₀). Blood immunophenotyping showed persistent B-cell aplasia, hypogammaglobulinemia (4.0 g/L), and CD4⁺ T-cell lymphopenia (0.3 × 10⁹/L) with a marked reduction in naïve CD4⁺ T-cells (2%).
Immune checkpoint blockade with nivolumab was initiated, combined with intravenous immunoglobulin. Treatment was well tolerated. After 3 cycles, JC viral load markedly decreased to a detectable but non-quantifiable level. MRI showed a dissociated response, with regression of all previous white matter lesions but progression of a left frontal lesion >1 cm (Figure 1). End treatment blood immunophenotyping showed persistent B-cell aplasia (0 × 10⁹/L) and CD4⁺ T-cell lymphopenia (0.38 × 10⁹/L) (Figure 2). One year after PML diagnosis and four years after CAR T-cell infusion, the patient showed significant cognitive function improvement, and remained alive with limited functional impairement.
Which immune abnormalities may have favored the development of progressive multifocal leukoencephalopathy in this patient?
A. CD4⁺ T-cell lymphopenia
B. Persistent B-cell aplasia
C. Hypergammaglobulinemia
D. Expansion of regulatory T cells
E. Neutrophilia
Expert Perspective by Dr Nicolas Gazeau
Progressive multifocal leukoencephalopathy (PML) is a severe opportunistic infection caused by JC virus reactivation in the setting of impaired cellular immunity. While CD4⁺ T-cell deficiency is a well-known predisposing factor in patients with HIV infection or solid-organ transplantation leading to impaired activation of JC virus–specific CD8⁺ T-cells; recent evidence has highlighted the role of prolonged B-cell aplasia to PML pathogenesis, particularly following anti-CD20 monoclonal antibody or CAR T-cell therapy1.
B cells play multiple roles in JC virus biology beyond antibody production. They represent a potential reservoir for latent infection and a vehicle for viral trafficking to the central nervous system. Sustained B-cell aplasia, as seen after CD19-directed therapies or prior anti-CD20 exposure, may therefore promote viral reactivation and hematogenous dissemination to neural tissues. Moreover, B-cell loss interrupts antigen presentation to CD4⁺ T cells and compromises the generation and maintenance of JCV-specific cytotoxic responses. The resulting collapse of both humoral and cellular antiviral immunity creates a permissive environment for PML development, as emphasized in mechanistic studies and immune-reconstitution analyses following CAR T-cell therapy.
PD-1 blockade with nivolumab can restore antiviral immunity in PML by reversing T-cell exhaustion and reactivating JC virus–specific CD8⁺ and CD4⁺ T-cell responses. This leads to enhanced cytokine production and cytotoxic function against infected glial cells.3.
This case highlights the risk of late-onset opportunistic infections following CAR T-cell therapy, particularly in patients with persistent B-cell aplasia and, in some cases, concomitant T-cell deficiency. The coexistence of these immune defects may persist for years after CAR T-cell therapy, maintaining a state of susceptibility to opportunistic infections.
Long-term B-cell aplasia has been inconsistently documented among CAR T-cell studies. In pivotal trials, the JULIET study found that half of the patients who achieved complete remission had detectable B cells at a median of 6.7 months following tisagenlecleucel infusion. Conversely, in the ZUMA-1 trial, B-cell recovery was observed in approximately 40% of patients at 12 months and in 75% at 24 months after axicabtagene ciloleucel.
Recently, case series and pharmacovigilance registry analyses have reported occurrences of PML following anti-CD19 CAR T-cell therapy1. In one study, 13 cases were identified in the FAERS (FDA Adverse Event Reporting System) and CIBMTR (Center for International Blood and Marrow Transplant Research) registries, corresponding to an estimated incidence of 0.9 per 1000 treated patients and a median time to onset of eight months1. In our patient, PD-1 blockade with nivolumab achieved a significant and encouraging virological and radiological response, given that mortality rates for PML after CAR T-cell therapy have been reported to approach 80% in some series1.
In conclusion, as an increasing number of patients achieve long-term remission or even cure after CD19-directed CAR T-cell therapy for lymphoma, careful evaluation of late and chronic toxicities has become essential. Long-term clinical and immunological follow-up is crucial to detect and manage delayed complications, particularly infectious ones. Prolonged B-cell aplasia, often accompanied by persistent hypogammaglobulinemia and incomplete T-cell recovery, remains a frequent finding and may predispose patients to opportunistic infections, including progressive multifocal leukoencephalopathy. These chronic immune alterations highlight the importance of comprehensive post–CAR T immune monitoring and individualized infection prophylaxis strategies.
This case emphasizes that restoring antiviral immunity through PD-1 blockade may represent a promising therapeutic strategy in carefully selected cases, leading to meaningful clinical improvement without major toxicity. Further investigation into mechanisms of immune reconstitution, risk stratification, vaccination strategies, and the management of prolonged B-cell aplasia will be key to improving long-term safety and outcomes in CAR T-cell survivors.
Correct Answers – A, B
References:
- Goldman et al. Progressive multifocal leukoencephalopathy in patients treated with chimeric antigen receptor T cells. Blood. 2023 Feb 9;141(6):673-677
- Tan CS et al. Progressive multifocal leukoencephalopathy and the spectrum of JC virus-related disease. Nat Rev Neurol. 2010 ;6(12):667–679.
- Mackenzie S et al. Pembrolizumab for the treatment of progressive multifocal leukoencephalopathy following anti-CD19 CAR-T therapy: a case report. EJHaem. 2021 Aug 4;2(4):848-853.
Future Clinical Case of the Month
If you have a suggestion for future clinical case to feature, please contact Anna Sureda.