February 2020 Clinical Case of the Month
Title: Pancytopenia 15 months after allogeneic hematopoietic cell transplantation for multiple myeloma
Submitted by: Syed Ali Abutalib and Nicolaus Kröger
Physicians expert perspective: Malgorzata Mikulska
A 52-year-old male patient from Germany who received allogeneic hematopoietic cell transplantation 15-months before for IgG kappa multiple myeloma after relapsing after 2 previous autografts complained about weakness and mild night sweat. Clinical examination was consistent with splenomegaly. Four weeks before he was on 2-week vacation to Mallorca Island.
His blood count showed pancytopenia with hemoglobin of 8 g/dL , WBC of 1.9 x109/L and platelets of 53 x109/L. Furthermore, IgG was elevated with 30g/dL and free light chain kappa was 452 mg/L consistent with serologic progression of multiple myeloma. Other elevated blood results were ferritin with 842µg/L and GGT with 204 U/L. HIV test was negative.
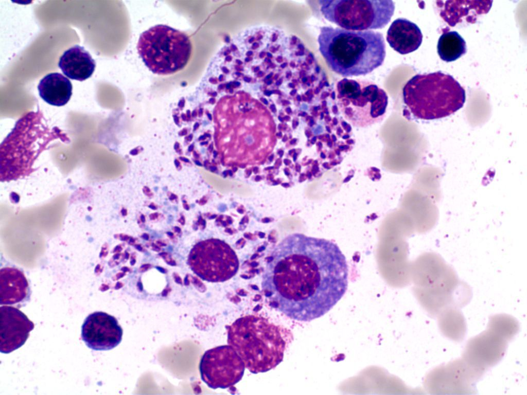
The bone marrow aspirate showed about 25% plasma cells and following picture on touch preparation.
Which of the following is the correct diagnosis?
- Secondary acute leukemia with Auer bodies
- Macrophage activation syndrome
- Intracellular histoplasma
- Hypereosinophilic syndrome
- Intracellular amastigotes
Expert Perspective by Malgorzata Mikulska
The picture does not show Auer bodies or eosinophils or signs of Macrophage activation syndrome, thus answer A, B and D is not correct. The picture of the bone marrow smears shows beside plasma cells, Leishmania amastigotes (round to oval non flagellated cells) inside macrophage and monocyte. Answer D is also incorrect: Histoplasmosis is an infection caused by a dimorphic fungus, Yeast forms of histoplasmosis can be seen intracellularly in bone marrow, and they have similar dimentions to leishmania amastigotes. However, they do not have a kinetoplast and are positive on fungal strains.
Leishmaniasis is a vector-borne disease caused by protozoa of the genus Leishmania. It is usually transmitted by sand fly bites, but it can be also transmitted by blood transfusions or solid organ transplantation. Clinical forms of leishmaniasis range from cutaneous and muco-cutaneous lesions to a systemic multiorgan disease, called visceral leishmaniasis.
Visceral leishmaniasis is endemic in Mediterranean region, including Southern Europe, and certain zones in South America, Asia and Africa. In the Mediterranean, visceral leishmaniosis is usually caused by L. infantum, while in eastern Africa, Bangladesh, India, and Nepal L. donovani is predominant. Patients with impaired cellular immunity are at increased risk of leishmaniasis, both of de novo infection and reactivation. While typical incubation period for symptomatic visceral leishmaniasis varies in median from 2 to 6 months, parasites may persist for long periods of time and cause reactivation in case of immunosuppression.
The signs, symptoms and laboratory findings of visceral leishmaniasis include fever, weakness, weight loss, hepatosplenomegaly, anemia, leukopenia, thrombocytopenia, pancytopenia, and hypergammaglobulinemia. Since these are nonspecific findings; they can be easily attributed to other conditions in allo-HCT recipients.
The diagnosis is obtained most frequently through bone marrow aspirate which shows amastigotes in tissue and have a sensitivity of 53% to 86% (round to oval non-flagellated cells measuring 2-4 micrometers found inside macrophages or monocytes). Other diagnostic methods are isolation of promastigotes in culture, available only in reference laboratories; Leishmania serology, which can have low sensitivity in the immunocompromised patients; urine antigen, which has variable performance; and molecular techniques. The advantages of PCR assays are high sensitivity and specificity (respectively, 92 to 93% and 63 to 96% in blood; 95 to 95% and 76 to 92% in bone marrow), the possibility to perform it in different samples (most frequently bone marrow aspirate, but also in the peripheral blood) and the ability to monitor the response to treatment.
Liposomal Amphotericin B is currently the treatment of choice, and multiple doses are recommended in immunocompromised hosts such as presented in this case. Typically, the treatment requires liposomal amphotericin B at a dose of 4 mg/ kg daily on days from 1 to 5, followed by single administrations on days 10, 17, 24, 31, and 38 for the total dose of 40 mg/kg.
Secondary prophylaxis is currently not recommended outside of HIV setting but relapses may occur.
Correct Answer – E
References
- Aronson N, Herwaldt BL, Libman M, Pearson R, Lopez-Velez R, Weina P, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the infectious diseases society of America (IDSA) and the American society of tropical medicine and hygiene (ASTMH). Am J Trop Med Hyg 2017;96:24e45.
- van Griensven J and Diro Ermias. Visceral Leishmaniasis: Recent Advances in Diagnostics and Treatment Regimens. Infect Dis Clin North Am 33 (2019) 79–99. https://doi.org/10.1016/j.idc.2018.10.005
- Jimenez-Marco T, Fisa R, Girona-Llobera E, Cancino-Faure B, et al. Transfusion-transmitted leishmaniasis: a practical review. Transfusion 2016;56(Suppl. 1):S45e51.
- de Ruiter CM, van der Veer C, Leeflang MM, et al. Molecular tools for diagnosis of visceral leishmaniasis: systematic review and meta-analysis of diagnostic test accuracy. J ClinMicrobiol 2014;52(9):3152
- Tatarelli P, Fornaro G, Del Bono V, Nicolini LA, Raiola AM, Gualandi F, et al. Visceral leishmaniasis in hematopoietic cell transplantation: Case report and review of the literature. J Infect Chemother. 2018 Dec;24(12):990-994. doi: 10.1016/j.jiac.2018.05.008
Contacts
Syed Ali Abutalib, MD
Associate Director, Hematology and Cellular Therapy Program
Director, Clinical Apheresis Program
Cancer Treatment Centers of America, Zion, Illinois
Associate Professor, Rosalind Franklin University of Medicine and Science
Email: abutalib110@gmail.com
Nicolaus Kröger, MD
Professor and Medical Director of the Department of Stem Cell
Transplantation at the University Hospital Hamburg-Eppendorf, Germany
University Hospital Hamburg, Hamburg, Germany
Correspondence: Nicolaus Kröger, MD
Email: nkroeger@uke.uni-hamburg.de
Expert Perspective
Professor Malgorzata Mikulska
Associated Professor of Infectious Diseases at the University of Genova, working at the Division of Infectious Diseases, IRCCSS Ospedale Policlinico San Martino in Genova, Italy.
EBMT Infectious Diseases Working Party Secretary.
m.mikulska@unige.it
Future Clinical Case of the Month
If you have a suggestion for future clinical case to feature, please contact Nicolaus Kröger.