January 2024 Clinical Case of the Month
Title: The Management of Multiple Myeloma in a Renal Transplant Recipient
Submitted by Dr. Uğur Şahin, Atılım University, Medicana International Ankara Hospital, Hematology and bone marrow transplantation unit, Ankara, Turkey.
Physicians expert perspective: Meral Beksaç, MD, Istinye University Ankara Liv Hospital Haematology and Stem Cell Transplantation Unit.
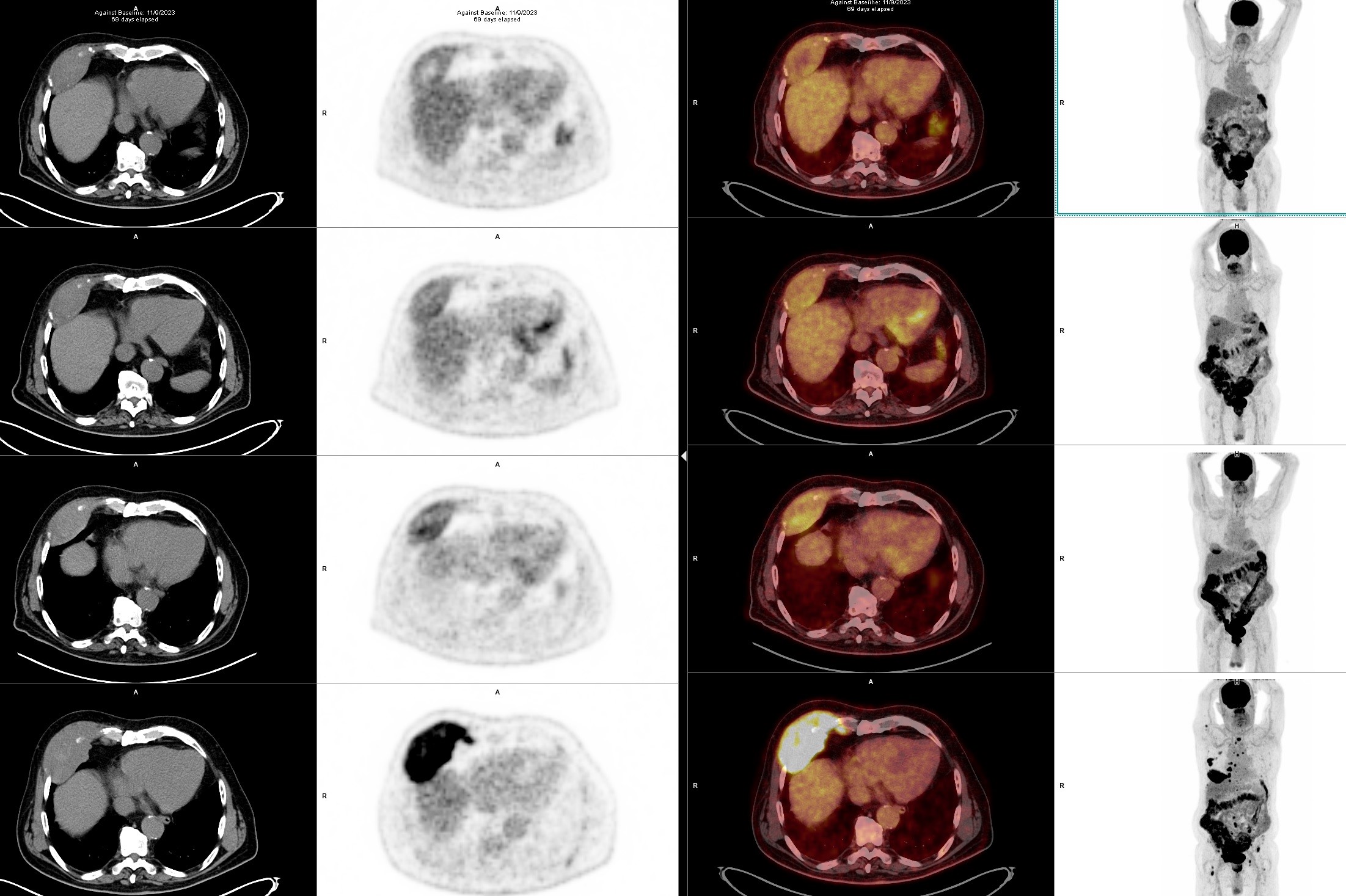
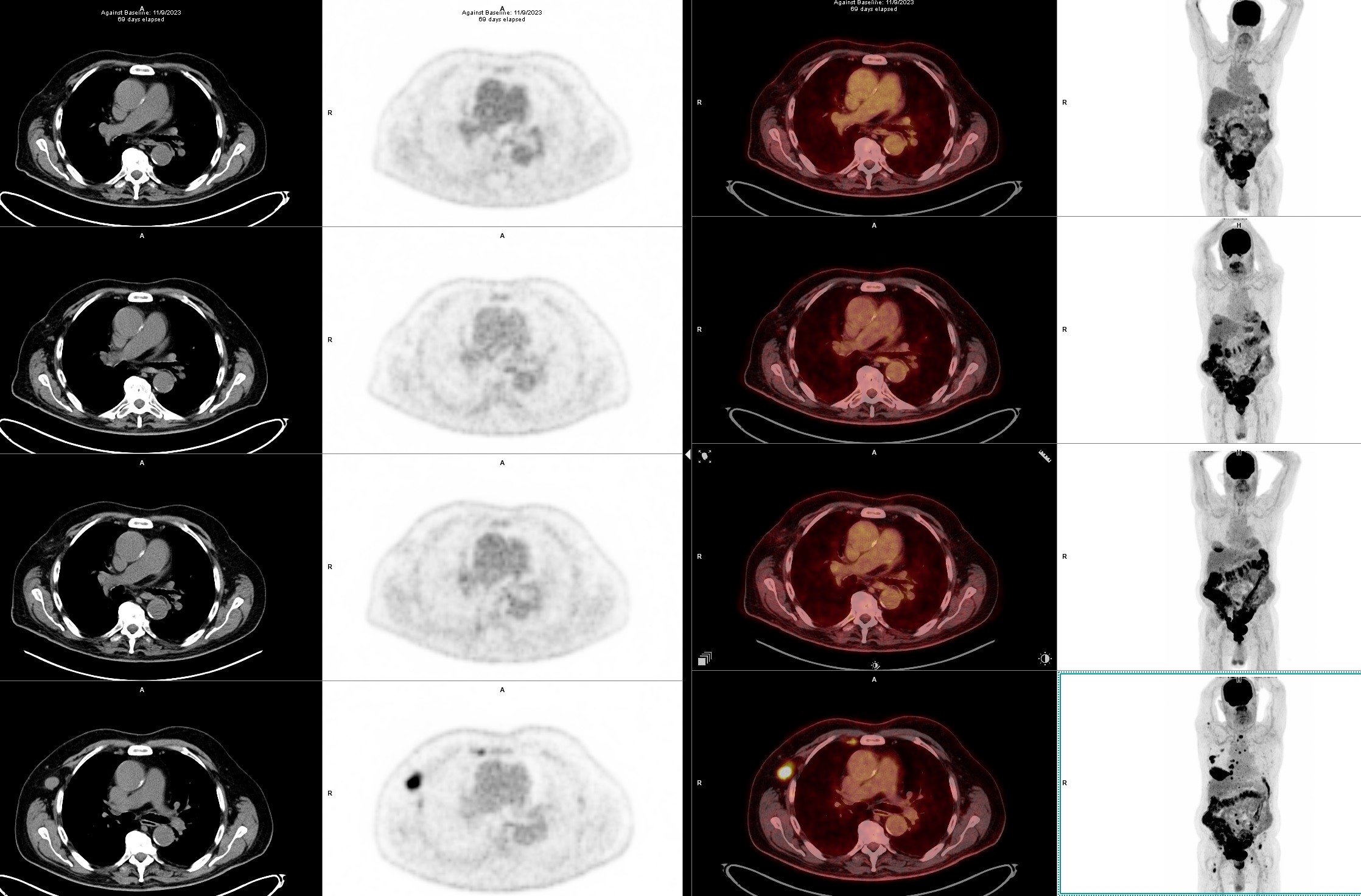
A 71-year-old male presented with a bone pain localized to the right hip. The magnetic resonance imaging (MRI) and positron emission tomography with computed tomography (PET-CT) (bottom image of the Figure 1a and 1b)scans revealed multiple lytic bone lesions together with some nodal and extramedullary pathological involvements, including an enlarged palpable axillary lymph node and a paraosseus lesion of 10 x 4 cm in size located on the anterior thorax. The unilateral bone marrow biopsy did not show a clonal plasma cell infiltration and thus FISH was normal. The lymph node excisional biopsy demonstrated 100% infiltration with lambda (+) clonal plasma cells, EBER (-), BRAF (V600E, H608L) and NF1 mutations (+) by next generation sequencing (NGS). The ratios of serum total and free light chain kappa/lambda were 2800/4470 mg/L and 22/25 mg/L, respectively, suggesting a monoclonal dyscrasia without any co-existing immunoparesis or urine excretion. The diagnosis of lambda light chain multiple myeloma (MM) of R-ISS stage 1 was confirmed.
The patient is a retired chemical engineer and has a history of end stage renal failure secondary to focal segmental glomerulosclerosis, which was treated with a kidney transplant from a 1/6 HLA-match family member in 2013. Prior to renal disease, he had a history of colon carcinoma diagnosed in 2004 and required chemotherapy which provided a continuous remission thereafter. He had also received treatment with radioactive iodine for thyroid papillary carcinoma in 2011. In 2022 he developed facial basal cell carcinoma which was surgically removed totally. Since he had a history of multiple malignancies and a reactivation of BK virus during his follow-up for renal transplant, immunosuppresive treatment was limited to cyclosporin and low dose Prednisolone only, under which he developed MM. He also had type 2 diabetes mellitus, which was under control with oral hypoglycemic agents. At myeloma presentation his hemogram and renal functions were within normal limits.
What is your choice for induction therapy?
a. VCD (bortezomib – cyclophosphamide – dexamethasone)
b. VRD (bortezomib – lenalidomide – dexamethasone)
c. Daratumumab + VDT (bortezomib – dexamethasone – thalidomide)
d. Daratumumab + VD (bortezomib – dexamethasone)
e. Daratumumab + RD (lenalidomide – dexamethasone)
Expert perspective by Meral Beksaç:
The treatmet decision for such a case of MM secondary to immunosuppression and two additional non-hematological cancers could hardly be based on evidence since such patients are excluded from clinical trials. Although there is a growing interest in lymphoma developing among renal transplant recipients (1, 2) there are few reports on MM developing during the course of renal transplantation (3-9). In these reports, extramedullary presentation is a frequent finding. Treatment of these cases have been heterogeneous (i.e.; melphalan, thalidomide, PAD alone, VRD or pomalidomide with or without autologous stem cell transplantation (ASCT) / radiotherapy (3). In a series of 3518 renal transplant cases among which twenty three had a pre-existing diagnosis of MGUS from Mayo clinic with a median post-transplant follow-up of 1783 days none developed treatment requiring MM (9).
Considering the high risk conferred by the extramedullary involvement (nodal and paraosseous) in this case, we decided to try a triplet combination with Dara + VD, which is known not to be nephrotoxic (10). Although the patient responded with a reduction in free light chain levels, the pain complaints did not resolve and even increased. The PET-CT scan after the second cycle (Figure 1a and 1b) revealed the disappearance of nodal and bone-related FDG uptakes, whereas a minor reduction in size/uptake was observed for the thoracal paraosseous disease. Subsequently, the treatment was switched to daratumumab + KD (carfilzomib – dexamethasone) as described in the CANDOR study (11). During the early follow-up, a decline in eGFR (estimated glomerular filtration rate) prompted us to split the carfilzomib dose to 20 mg/m2 twice weekly, which reversed the renal impairment. A transient hypertension up to 150/95 mm Hg has accompanied each carfilzomib infusion. The patient has currently completed the fourth cycle with a complete hematologic and metabolic response, however, without a reduction in the size of the paraosseous lesion (Figure 1a and 1b). As an attempt to reduce immunosuppression the patient was already under two drug regimen at diagnosis instead of three drug regimen and cyclosporine levels are kept below the 100 ng/mL marginal range.
The negativity of EBV in this case suggests a plasma dyscrasia arising from the recipient rather than a donor derived clone (12). BRAF mutation is a frequent abnormality among myeloma patients. Two distinct mutations on the same exon with either gain or loss of function have been observed in this case (13).
Our future plan will be to continue daratumumab + KD regimen with conversion to a maintenance phase with less frequent intervals. ASCT is not scheduled in near future due to potential risks related to his age and renal transplantation status.
References:
- Patil R, Prashar R, Patel A. Heterogeneous Manifestations of Posttransplant Lymphoma in Renal Transplant Recipients: A Case Series. Transplant Proc 2021;53:1519-27.
- Zhang J, Ma L, Xie Z, et al. Epidemiology of post-transplant malignancy in Chinese renal transplant recipients: a single-center experience and literature review. Med Oncol 2014;31:32.
- Safadi S, Dispenzieri A, Amer H, et al. Multiple myeloma after kidney transplantation. Clin Transplant 2015;29:76-84.
- Sharma S, Rana C, Vinod PB, Gupta A. Plasma cell myeloma in a renal transplant recipient: A case report and review of literature. Indian J Nephrol 2011;21:270-2.
- Howard AD, Moore J, Jr., Tomaszewski MM. Occurrence of multiple myeloma three years after successful renal transplantation. Am J Kidney Dis 1987;10:147-50.
- Fujiwara SI, Ikeda T, Morita K, et al. Multiple myeloma derived from a kidney transplant donor who also developed myeloma after kidney donation. Am J Transplant 2019;19:2374-7.
- Bhasin B, Szabo A, Wu R, et al. Monoclonal Gammopathies After Renal Transplantation: A Single-center Study. Clin Lymphoma Myeloma Leuk 2020;20:e468-e73.
- Aslam N, Trautman CL, Sher T. Kidney Transplantation and Monoclonal Gammopathy of Undetermined Significance. Transplant Direct 2021;7:e723.
- Naina HVK, Kyle R, Habermann TM, et al. Pre Transplantation MGUS and Transformation to Multiple Myeloma in Kidney Transplantation: A Single Center Experience. Blood 2007;110:4779.
- Beksac M, Kroger N, Byrne JL, Ganser A, Yegin ZA, Schonland S. Allogeneic hematopoietic cell transplantation (allo-HCT) outcomes in myeloma patients on renal replacement therapy: a report from the Chronic Malignancy Working Party (CMWP) of the European Society of Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant 2021;56:529-31.
- Usmani SZ, Quach H, Mateos MV, et al. Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): updated outcomes from a randomised, multicentre, open-label, phase 3 study. Lancet Oncol 2022;23:65-76.
- Peri N, Kussick S, Bakthavatsalam R, Mitsumori L, Dighe M. Postrenal transplant non-EBV multiple myeloma of donor origin. Am J Transplant 2006;6:419-22.
- Cengiz Seval G, Kuzu I, Yuksel S, et al. Next Generation Sequencing May be Helpful in Designing Novel Combinations Among Heavily Pretreated Myeloma Patients, Refractory to All Available Approved Anti-Myeloma Drugs. Blood 2019;134:3197-.
Acknowledgements: We are indepted to Drs Gülşah Kaygusuz, Işınsu Kuzu, Haldun Umudum (pathologists), Seyfettin Ilgan (nuclear medicine specialist) for their most valuable expertise in the molecular diagnosis and imaging response assessments.
Future Clinical Case of the Month
If you have a suggestion for future clinical case to feature, please contact Anna Sureda.