November 2023 Clinical Case of the Month
Title: Early T-cell expansion post T-replete mismatched cord transplant with granulocytes is associated with induction of remission and sustenance in relapsed-refractory high risk acute myeloid leukemia.
Submitted by:
Dr. Abdul Moothedath, MD, DM (Haematology)
Dr. Srividhya Senthil, MD, MRCPCH, FRCPath (Haematology)
Physicians expert perspective:
Professor Rob Wynn, MB BChir, MD, MRCP, FRCPath (Haematology)
Director of Bone Marrow Transplant and Cell Therapies, Royal Manchester Children’s Hospital, United Kingdom.
A 6-year-old boy with relapsed refractory AML post cord blood transplant was referred to have a second cord blood stem cell transplant under the clinical trial called “GRANS trial “. He was originally diagnosed at 3 years of age with high risk disease with t(16:12), FUS:ERG fusion (which was then classified as intermediate risk) treated with standard AML chemotherapy. Soon after, he had a relapsing molecular disease which was refractory to chemotherapy including FLA-Ida and azacitidine. He had a busulphan based myeloablative cord blood stem cell transplant with a 6/8 mismatched cord blood. He received ATG for in vivo T cell depletion.
Conforming with an aggressive disease, there was a frank disease relapse immediately after the transplant, which failed to respond to bridging treatment including venetoclax.
In such an intractable, chemo refractory, early post-transplant relapsed disease, where palliative treatment was seemingly the only available option, he was offered an experimental approach with GRANS under which, he was given a T replete, 6/8 mismatched unrelated cord blood stem cell transplant with the administration of third-party pooled granulocytes around the D0. Fludarabine, Treosulphan and Thiotepa conditioning was used, cislosporin and mycophenolate mofetil (MMF) were used for GVHD prophylaxis. He had evidence of preexisting fungal chest infection which was treated with Voriconazole and Micafungin. He had cardiac dysfunction which was treated with carvedilol.
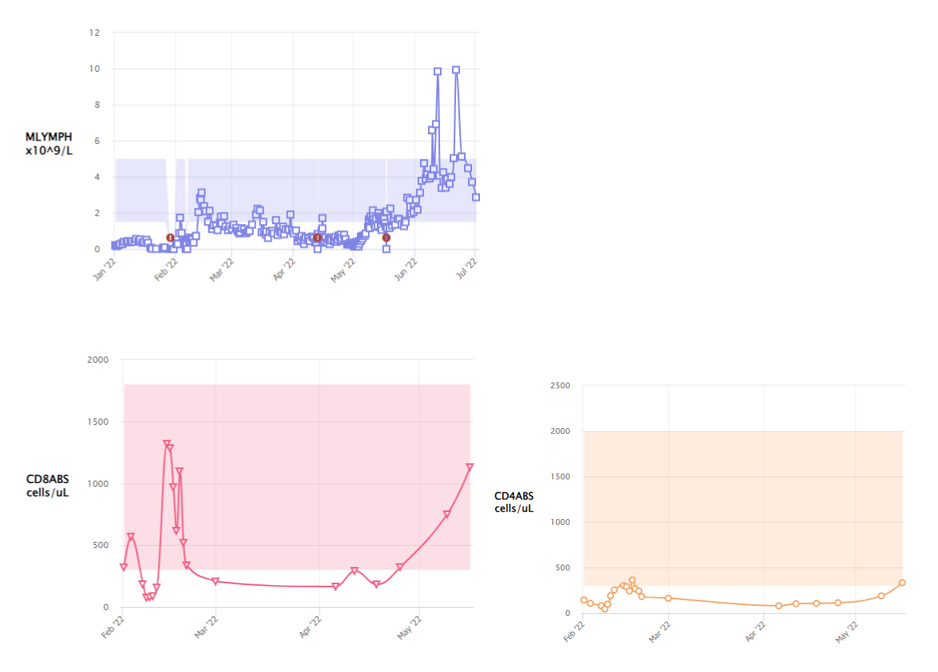
On Day 7 of stem cell transplant, he developed persistent, prolonged, high-grade fevers, oxygen requirement necessitating intensive care support and raised inflammatory markers with a CRP of 430mg/L and Serum Procalcitonin of 19mcg/L. Routine blood cultures were negative. Nasopharyngeal aspirate was negative for respiratory viruses by PCR. PCR for adenovirus, CMV and EBV were negative in blood. X-ray chest showed bilateral patchy changes and small pleural effusion. This episode paralleled with a massive CD8 T- lymphocyte expansion (CD3-663, CD4 106, CD8-568 cells/microliter) following granulocyte infusions, which peaked at day +10 post HSCT. (Figure 1). This was followed by neutrophil engraftment on day +19.
Which of the following is the most probable cause of prolonged fever in this boy and what is the further management to be considered?
A) Progression of pre-existing fungal chest infection and consider broncho alveolar lavage and change of antifungals.
B) Engraftment syndrome and consider administration of steroids.
C) Viral reactivation and send extended screen for viral PCRs to guide antiviral treatment.
D) Cytokine release syndrome following granulocyte infusion and consider anti IL-6 antibody or steroids if further deterioration.
E) Infective Endocarditis with background cardiomyopathy and perform echocardiogram to rule out vegetations.
Correct Answer - D
Expert opinion:
The above findings are typically seen in CRS associated with granulocytes administration during a T replete cord blood transplant.
T-replete mismatched cord blood transplant (TRCB) offers an augmented graft versus leukemia (GVL) effect mediated by alloreactive donor T cells and thereby presents a potential for long term remission and cure in high risk and relapsed refractory AML in children. Easy access for urgent transplants, greater tolerance of HLA mismatch and very less rate of chronic GVHD are other advantages of cord blood. (1) In a multi-centric retrospective analysis of children who received stem cell transplant for high-risk AML, TRCB cohort had significantly improved event free survival and time to relapse and overall survival compared to the children who received stem cell transplant from other sources. (2)
One of the methods to enhance GVL effect of cord blood transplant (CBT) is by using third party pooled granulocyte transfusions which instigates and supports mismatched HLA antigen presentation to the donor immune system, and thereby promotes early cytotoxic CD8+ T cell expansion, which is exploited in improving outcomes in GRANS trial. Albeit the predominant T cell type being CD4+ in the cord blood units, with granulocyte transfusion following CBT, there is a specific expansion of donor CD8+ T cells with a switch from naïve to effector memory phenotype that exhibit cytotoxicity. (3) We also endeavor to wean the GVHD prophylaxis early, in the absence of GVHD, to offer an augmented GVL.
In our experience, the peak expansion of donor T lymphocytes occurred at a median of 9 days post-transplant. Following transient initial expansion, their numbers fell to be replaced by a corresponding increase in CD4+ cells. This is contrary to what’s observed in CBT without granulocytes, where the lymphocyte expansion occurs late and is predominantly CD4+ T cells. (3, 4) Cytokine release syndrome (CRS) was almost seen in all the patients who received granulocytes and the symptoms correlated with the peak of lymphocyte expansion. Severe CRS required steroids or IL-6 blockade to calm down the inflammatory response.
We have published our experience with granulocytes and CBT treating 10 patients with high-risk AML including those who relapsed after a previous transplant. 80% achieved MRD negativity and 50% are alive and disease free after a median follow up of 12.7 months. (5) The early results are encouraging and offers a hope of cure in this high-risk cohort with dismal outcomes. However, randomized studies of CBT with or without granulocytes with longer follow up period are needed to elucidate the immunological properties and outcomes.
The above patient was treated with a short course of steroid after which the inflammatory response dampened. MMF was stopped by D20 and ciclosporin was weaned and stopped by D30. His transplant course was further complicated by development of severe grade 3, acute, gut and skin GVHD. This was managed with prolonged immunosuppressive treatments including steroids, infliximab, ruxolitinib and extracorporeal photopheresis. He also had evidence of transplant associated microangiopathy with complement activation around 2 months post HSCT, which was successfully treated with complement inhibition. As a result of immunosuppressive treatment, he had CMV reactivation requiring treatment with multiple antivirals including ganciclovir and foscarnet.
Currently, he is 22 months post-transplant and remains fully donor cell engrafted with good immune reconstitution. He continues to be in MRD negative remission from high-risk AML, without evidence of chronic GVHD.
References:
- Wynn R, Nataraj R, Nadaf R, Poulton K, Logan A. Strategies for success with umbilical cord haematopoietic stem cell transplantation in children with malignant and non-malignant disease indications. Front Cell Dev Biol. (2022) 10. doi: 10.3389/ fcell.2022.836594.
- Horgan C, Mullanfiroze K, Rauthan A, Patrick K, Butt NA, Mirci-Danicar O, et al. T-replete cord transplants give superior outcomes in high risk and relapsed/ refractory paediatric myeloid malignancy. Blood Adv. (2023) 7(10):2155–65. doi: 10. 1182/bloodadvances.2022009253.
- Borrill R, Poulton K and Wynn R (2023) Immunology of cord blood T-cells favors augmented disease response during clinical pediatric stem cell transplantation for acute leukemia. Front. Pediatr. 11:1232281. doi: 10.3389/fped.2023.1232281.
- Hiwarkar P, Adams S, Gilmour K, Nataraj R, Bonney D, Poulton K, et al. Cord blood CD8+ T-cell expansion following granulocyte transfusions eradicates refractory leukemia. Blood Adv. (2020) 4(17):4165–74. doi: 10.1182/bloodadvances. 2020001737.
- Borrill R, Poulton K, Kusyk L, Routledge A, Bonney D, Hanasoge-Nataraj R, et al. Granulocyte transfusion during cord blood transplant for relapsed, refractory AML is associated with massive CD8+ T-cell expansion, significant cytokine release syndrome and induction of disease remission. Br J Haematol. (2023) 202(3):589–98. doi: 10.1111/bjh.18863.
Future Clinical Case of the Month
If you have a suggestion for future clinical case to feature, please contact Anna Sureda.