January 2023 Clinical Case of the Month
Title: Donor Lymphocyte Infusion for EBV reactivation as bridging therapy for second Allogeneic Stem Cell Transplantation in EBV Driven Angioimmunoblastic Lymphoma
Submitted by: Dr Charalampia Kyriakou MD, PhD
Physicians expert perspective: Dr Charalampia Kyriakou MD, PhD
Consultant Haematologist
Department of Haematology
University College London Hospital
London, United Kingdom
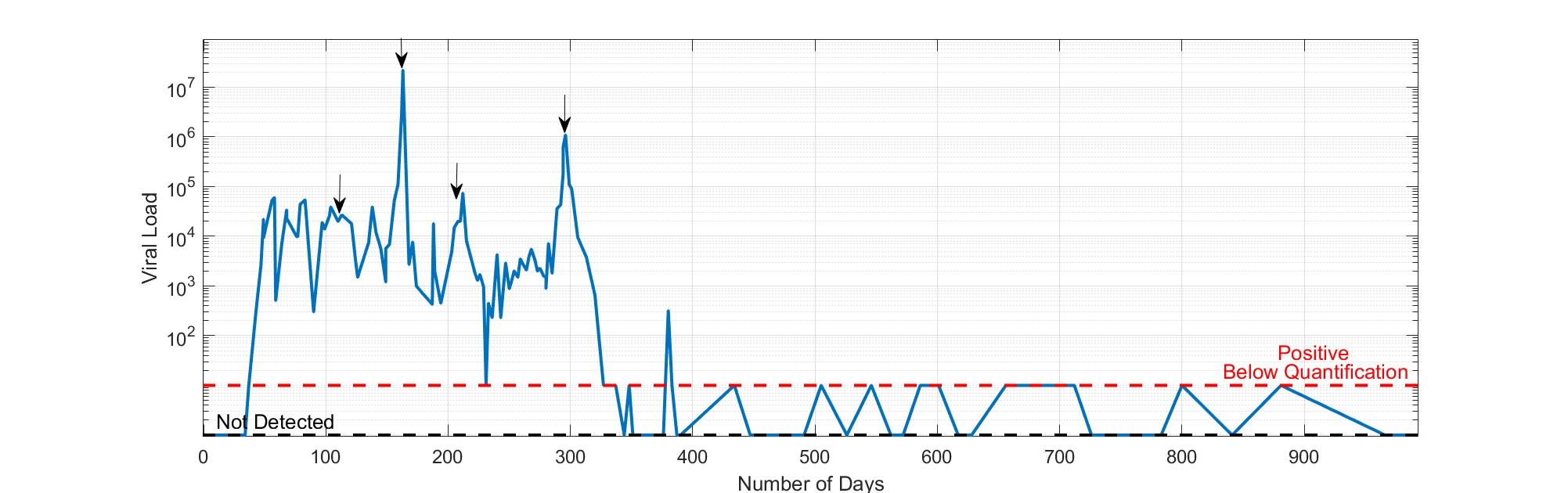
A 47-year-old gentleman was diagnosed with Stage IVSB EBV driven Angioimmunoblastic Lymphoma (AITL) and associated angioedema in July 2018. He received 3 cycles of RCHOEP and 1 cycle of salvage R-IVE regimen for progressive EBV driven AITL. Following each cycle after short lived transient response, the EBV titre was rapidly rising with associated systemic and nodal disease progression. He achieved complete remission following 6 doses of Brentuximab Vedotin (BV) anti-CD30 antibody and he underwent BEAM CAMPATH sibling Reduced Intensity Allogeneic Stem Cell Transplantation (alloSCT) in February 2019. He developed skin rash and progressive lymphadenopathy, 2 months following allo-SCT, without any evidence of GVHD. The PCR for EBV showed EBV reactivation, lymph node biopsy relapse AITL and chimerism analysis showed mixed chimerism. The immunosuppressive GVHD prophylaxis was discontinued and he received Rituximab without any response. He had donor lymphocyte infusion (DLI) at 1*10 ^7/kg in June 2019 with transient systemic, clinical and laboratory EBV titre response. He had further rising of the EBV titre to 22,000,000 DNA copies/ml with severe associated multiorgan failure and clinical deterioration. He was transferred to Intensive Care Unit requiring support for pneumonitis, pericarditis, hepatitis, colitis, renal failure and DIC. He continued deteriorating and he received a 2nd dose of DLI at 3*10^7/kg on the 1st of August 2019. Although the patient continued deteriorating further with additional meningitis symptoms, on renal dialysis and inotropic support, 5 days following the 2nd DLI, the EBV level was dramatically dropped from 22,000,000 DNA copies/ml to 2700 DNA copies/ml and the patient gradually recovered and became dialysis and ICU independent 20 days later. Due to his ECOG PS and until further clinical improvement, he received a 3rd dose of DLI at 3*10^7/kg on the 17th of September 2019 when EBV reactivation titre was raised at 15000 DNA copies/ml. Restaging PET CT after the 3rd DLI dose showed complete metabolic remission but he had trilineage cytopenia and the chimerism analysis showed graft rejection. The patient received a 4th dose of DLI on the 13th of December 2019 as bridging EBV approach prior to a 2nd allo-SCT, performed on the 10th of January 2020 using the same sibling donor with melphalan, fludarabine conditioning. The patient achieved and has sustained complete metabolic remission, without evidence of EBV reactivation, full donor chimerism and he is now back to full time job.
Which of the following is the most appropriate post-allogeneic transplant therapy for relapse of EBV driven AITL?
A. Palliative supportive therapy
B. EBV-specific T cell immunotherapy for rituximab-refractory EBV-associated lymphoma
C. Donor Lymphocyte Infusion
D. Donor Lymphocyte Infusion or EBV specific cellular therapy and second Allogeneic Stem Cell Transplantation
Expert Perspective by Dr Charalampia Kyriakou
Angioimmunoblastic T-cell lymphoma (AITL) accounts for 1-2% of all non-Hodgkin Lymphomas and it is the second most common subtype, representing 15%- 20%, of the peripheral T-cell lymphomas. AITL is characterised by complex clinicopathologic, genetic features and 80% to 95% of the cases are positive for Epstein-Barr virus (EBV) (1-3). The treatment can be challenging due to refractory progressive disease or early relapse after initial or subsequent therapy. The front-line treatment is CHO(E)P-based induction (4) with or without Autologous Stem Cell Transplantation (ASCT) consolidation (5, 6). ASCT is unlikely to sustain long-term response for patients with relapsed/refractory AITL and other PTCLs with disappointing outcomes when ASCT was used for relapsed/refractory PTCL (7-9) . More promising outcomes were observed with alloSCT, which was associated with a 2-5-year PFS of 45% to 53% for nodal PTCLs and as high as 81% for AITL (10, 11). Therefore, for allo-SCT eligible patients, consolidation with alloSCT should be considered if disease control can be obtained.
The critical question is which treatment approach should be used to achieve the disease control in the relapsed/refractory/progressive setting and to bridge to alloSCT? The treatment choices could include chemotherapy-based salvage with for example ICE (ifosfamide, carboplatin, and etoposide), which is associated with response rates up to 70% and potentially bridge patients to transplantation although this would not be expected to sustain disease control. Another option that could influence outcome for a subset of patients with AITL, is the brentuximab vedotin (BV), an anti-CD30 antibody drug conjugate. CD30 positivity was observed in 43% - 90% of patients with AITL. In a phase II study, BV achieved responses in 54% of patients with CD30-positive relapsed/refractory AITL (12-16). Other treatment options if available or within clinical trials, include histone deacetylase inhibitors that seem to have preferential activity in this disease, likely related to the high frequency of mutations in epigenetic modifier genes observed in AITL (17, 18). Other agents used include alemtuzumab, romidepsin, bortezomib, and belinostat (19-23).
EBV driven AITL is rare but very aggressive disease that although is more common in children it has been reported in adults. Haematopoietic stem cell transplantation remains the only curative therapy, although relapse of the disease after transplantation can also occur, and the use of donor-derived virus specific T cells could help to treat the relapses (Figure 1). It is critical, to diagnose and initiate transplantation before rapid disease progression to an irreversible stage. Treatment to temporarily reduce systemic toxicity associated with T-cell driven process might allow the patient time to receive a transplant. In the reported case the patient was diagnosed with EBV driven refractory to conventional therapy and achieved transient response with the anti-CD30 antibody. The application of alloSCT in this setting may result in better survival outcome (24). The EBV reactivation following relapse post alloSCT can be managed with the use of EBV specific adoptive T-Cell therapy (25-27) but could require time to be available. The timing of the treatment is important in a rapidly relapse progressive EBV driven lymphoma. The therapeutic benefit with the use of DLI has been reported in case series reports (28-33). The advantage of DLI was that, the cells were stored and were readily available to transiently control the EBV reactivation. The DLI infusions approach, enabled bridging to the second alloSCT and to a sustained complete remission afterwards.
References
- Xie Y, Jaffe ES. How I Diagnose Angioimmunoblastic T-Cell Lymphoma. Am J Clin Pathol. 2021;156(1):1-14.
- de Leval L, Parrens M, Le Bras F, Jais JP, Fataccioli V, Martin A, et al. Angioimmunoblastic T-cell lymphoma is the most common T-cell lymphoma in two distinct French information data sets. Haematologica. 2015;100(9):e361-4.
- Vose J, Armitage J, Weisenburger D, International TCLP. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124-30.
- Schmitz N, Trumper L, Ziepert M, Nickelsen M, Ho AD, Metzner B, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116(18):3418-25.
- d'Amore F, Relander T, Lauritzsen GF, Jantunen E, Hagberg H, Anderson H, et al. Up-front autologous stem-cell transplantation in peripheral T-cell lymphoma: NLG-T-01. J Clin Oncol. 2012;30(25):3093-9.
- Reimer P, Rudiger T, Geissinger E, Weissinger F, Nerl C, Schmitz N, et al. Autologous stem-cell transplantation as first-line therapy in peripheral T-cell lymphomas: results of a prospective multicenter study. J Clin Oncol. 2009;27(1):106-13.
- Smith SM, Burns LJ, van Besien K, Lerademacher J, He W, Fenske TS, et al. Hematopoietic cell transplantation for systemic mature T-cell non-Hodgkin lymphoma. J Clin Oncol. 2013;31(25):3100-9.
- Smith SD, Bolwell BJ, Rybicki LA, Brown S, Dean R, Kalaycio M, et al. Autologous hematopoietic stem cell transplantation in peripheral T-cell lymphoma using a uniform high-dose regimen. Bone Marrow Transplant. 2007;40(3):239-43.
- Kyriakou C, Canals C, Goldstone A, Caballero D, Metzner B, Kobbe G, et al. High-dose therapy and autologous stem-cell transplantation in angioimmunoblastic lymphoma: complete remission at transplantation is the major determinant of Outcome-Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2008;26(2):218-24.
- Le Gouill S, Milpied N, Buzyn A, De Latour RP, Vernant JP, Mohty M, et al. Graft-versus-lymphoma effect for aggressive T-cell lymphomas in adults: a study by the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire. J Clin Oncol. 2008;26(14):2264-71.
- Kyriakou C, Canals C, Finke J, Kobbe G, Harousseau JL, Kolb HJ, et al. Allogeneic stem cell transplantation is able to induce long-term remissions in angioimmunoblastic T-cell lymphoma: a retrospective study from the lymphoma working party of the European group for blood and marrow transplantation. J Clin Oncol. 2009;27(24):3951-8.
- Onaindia A, Martinez N, Montes-Moreno S, Almaraz C, Rodriguez-Pinilla SM, Cereceda L, et al. CD30 Expression by B and T Cells: A Frequent Finding in Angioimmunoblastic T-Cell Lymphoma and Peripheral T-Cell Lymphoma-Not Otherwise Specified. Am J Surg Pathol. 2016;40(3):378-85.
- Sabattini E, Pizzi M, Tabanelli V, Baldin P, Sacchetti CS, Agostinelli C, et al. CD30 expression in peripheral T-cell lymphomas. Haematologica. 2013;98(8):e81-2.
- Horwitz SM, Advani RH, Bartlett NL, Jacobsen ED, Sharman JP, O'Connor OA, et al. Objective responses in relapsed T-cell lymphomas with single-agent brentuximab vedotin. Blood. 2014;123(20):3095-100.
- Horwitz S, O'Connor OA, Pro B, Illidge T, Fanale M, Advani R, et al. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet. 2019;393(10168):229-40.
- Jagadeesh D, Horwitz S, Bartlett NL, Kim Y, Jacobsen E, Duvic M, et al. Response to Brentuximab Vedotin by CD30 Expression in Non-Hodgkin Lymphoma. Oncologist. 2022;27(10):864-73.
- Broccoli A, Argnani L, Zinzani PL. Peripheral T-cell lymphomas: Focusing on novel agents in relapsed and refractory disease. Cancer Treat Rev. 2017;60:120-9.
- Casulo C, O'Connor O, Shustov A, Fanale M, Friedberg JW, Leonard JP, et al. T-Cell Lymphoma: Recent Advances in Characterization and New Opportunities for Treatment. J Natl Cancer Inst. 2017;109(2).
- Coiffier B, Federico M, Caballero D, Dearden C, Morschhauser F, Jager U, et al. Therapeutic options in relapsed or refractory peripheral T-cell lymphoma. Cancer Treat Rev. 2014;40(9):1080-8.
- Moskowitz AJ, Horwitz SM. Targeting histone deacetylases in T-cell lymphoma. Leuk Lymphoma. 2017;58(6):1306-19.
- O'Connor OA, Horwitz S, Masszi T, Van Hoof A, Brown P, Doorduijn J, et al. Belinostat in Patients With Relapsed or Refractory Peripheral T-Cell Lymphoma: Results of the Pivotal Phase II BELIEF (CLN-19) Study. J Clin Oncol. 2015;33(23):2492-9.
- Sawas A, Ma H, Shustov A, Hsu P, Bhat G, Acosta M, et al. Characterizing the belinostat response in patients with relapsed or refractory angioimmunoblastic T-cell lymphoma. Leuk Lymphoma. 2020;61(8):2003-7.
- Stuver R, Epstein-Peterson ZD, Johnson WT, Khan N, Lewis N, Moskowitz AJ, et al. Current Treatment of Peripheral T-cell Lymphoma. Oncology (Williston Park). 2022;36(5):293-305.
- Kim TY, Min GJ, Jeon YW, Park SS, Park S, Shin SH, et al. Impact of Epstein-Barr Virus on Peripheral T-Cell Lymphoma Not Otherwise Specified and Angioimmunoblastic T-Cell Lymphoma. Front Oncol. 2021;11:797028.
- Sinha D, Srihari S, Beckett K, Le Texier L, Solomon M, Panikkar A, et al. 'Off-the-shelf' allogeneic antigen-specific adoptive T-cell therapy for the treatment of multiple EBV-associated malignancies. J Immunother Cancer. 2021;9(2).
- Heslop HE, Sharma S, Rooney CM. Adoptive T-Cell Therapy for Epstein-Barr Virus-Related Lymphomas. J Clin Oncol. 2021;39(5):514-24.
- McLaughlin LP, Bollard CM, Keller MD. Adoptive T Cell Therapy for Epstein-Barr Virus Complications in Patients With Primary Immunodeficiency Disorders. Front Immunol. 2018;9:556.
- Shimasaki N, Mori T, Shimada H, Sugita M, Higuchi M, Mukai M, et al. Epstein-Barr virus-associated posttransplant lymphoproliferative disorder after a cord blood stem cell transplantation presenting with pulmonary nodules. J Pediatr Hematol Oncol. 2004;26(2):124-7.
- Papadopoulos EB, Ladanyi M, Emanuel D, Mackinnon S, Boulad F, Carabasi MH, et al. Infusions of donor leukocytes to treat Epstein-Barr virus-associated lymphoproliferative disorders after allogeneic bone marrow transplantation. N Engl J Med. 1994;330(17):1185-91.
- Bollard CM, Rooney CM, Heslop HE. T-cell therapy in the treatment of post-transplant lymphoproliferative disease. Nat Rev Clin Oncol. 2012;9(9):510-9.
- El-Bietar J, Bollard C. T-cell therapies for Epstein-Barr virus-associated lymphomas. Pediatr Hematol Oncol. 2011;28(8):627-39.
- Bollard CM, Cohen JI. How I treat T-cell chronic active Epstein-Barr virus disease. Blood. 2018;131(26):2899-905.
- Bollard CM, Heslop HE. T cells for viral infections after allogeneic hematopoietic stem cell transplant. Blood. 2016;127(26):3331-40.
Future Clinical Case of the Month
If you have a suggestion for future clinical case to feature, please contact Anna Sureda.