The EBMT Registry

In 2023 we reached a major milestone for EBMT. On August 24, 2023, the EBMT officially launched its new and improved patient registry, co-funded by the European Union under the EuroTraCTOR grant. This is a remarkable achievement reflecting several years of dedicated teamwork. For more than 20 years, the EBMT has worked with ProMISe; a data-entry and registry solution. For some time, the EBMT has searched for a new solution that meets current and future data collection and retrieval needs and adjusts to innovative treatments and associated legal and technical requirements. The EBMT has now successfully established the next generation software, a successor to ProMISe, that will significantly improve the level of science and facilitate studies.
At launch of the EBMT Registry, the clinical data of almost 700,000 patients, who have undergone HCT, received immunosuppressive or cellular therapies have been migrated from ProMISe and Castor to the new platform. This process has continued after the launch and will continue during the spring of 2024 until all core data has been migrated into the new Registry.
Starting in August 2023, all Registry users were required to complete the mandatory e-learning to obtain certification before gaining access to the new EBMT Registry database. Since the go-live date, over 500 users have been successfully activated in the new EBMT Registry.
In addition, the EBMT Registry Helpdesk Team started to host weekly online Q&A sessions and medical sessions to provide support, address concerns, and promptly respond to the questions of the registered users.
The EBMT Registry is in ongoing development. Releases of new versions have been launched in 2023, adding new functionalities, improvements of existing functionalities, and bug fixes. Since the first release on August 24, three new versions have been launched (September 26, October 30, December 4). The December release introduced patient notes, filtering on question statuses, and more. Read everything about the versions on the webpage Registry Release Updates.
The collaboration between EBMT and the National Registries has been key in this designing phase. Regular monthly meetings were organised with National Registries.
In December the new Registry Committee with representatives from the various stakeholders including internal key staff started their activities with a Kick off meeting. Monthly calls will be planned for this committee.
A very large amount of work has been performed by many EBMT employees during this process with support of the working parties and there is still much work to do during 2024. Hopefully, this will result in a registry platform that will facilitate the key goals of the EBMT. We are grateful for all the work from all involved.

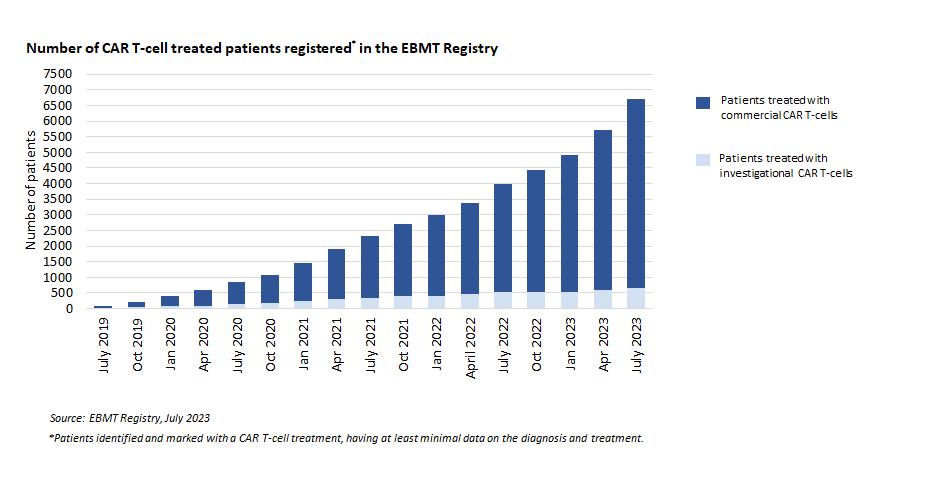
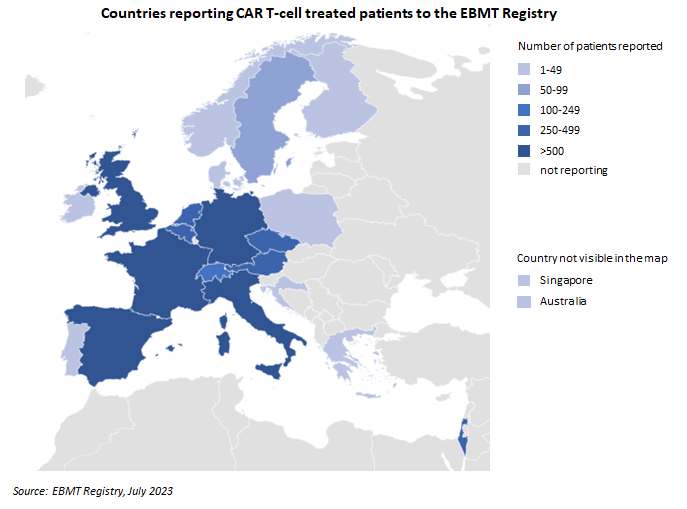
Registrations for Cellular Therapy