The PDWP aims to support research and education to improve the availability, safety, and efficacy of HSCT and other cellular therapeutics for children and adolescents; to engage and promote active co-operation with all EBMT working parties treating children and adolescents in an effort to meet the total needs of the full spectrum of paediatric HSCT patients; to initiate and perform prospective, collaborative, GCP-conform studies for malignant and non malignant paediatric diseases; to develop further the close collaboration with experts from the Leukaemia/Chemotherapy national frontline studies in order to define the optimal timing of HSCT and incorporate transplant recommendations into the disease-specific protocols; to implement the new regulations on paediatric medicines from the EMEA; to offer physicians and nursing staff from small or new centres practical training and fellowships in experienced transplantation units through a European Collaborative Paediatric HSCT network; to further develop established Paediatric Standards within the Accreditation Process through JACIE to guarantee and maintain a high quality of patient care and experience.
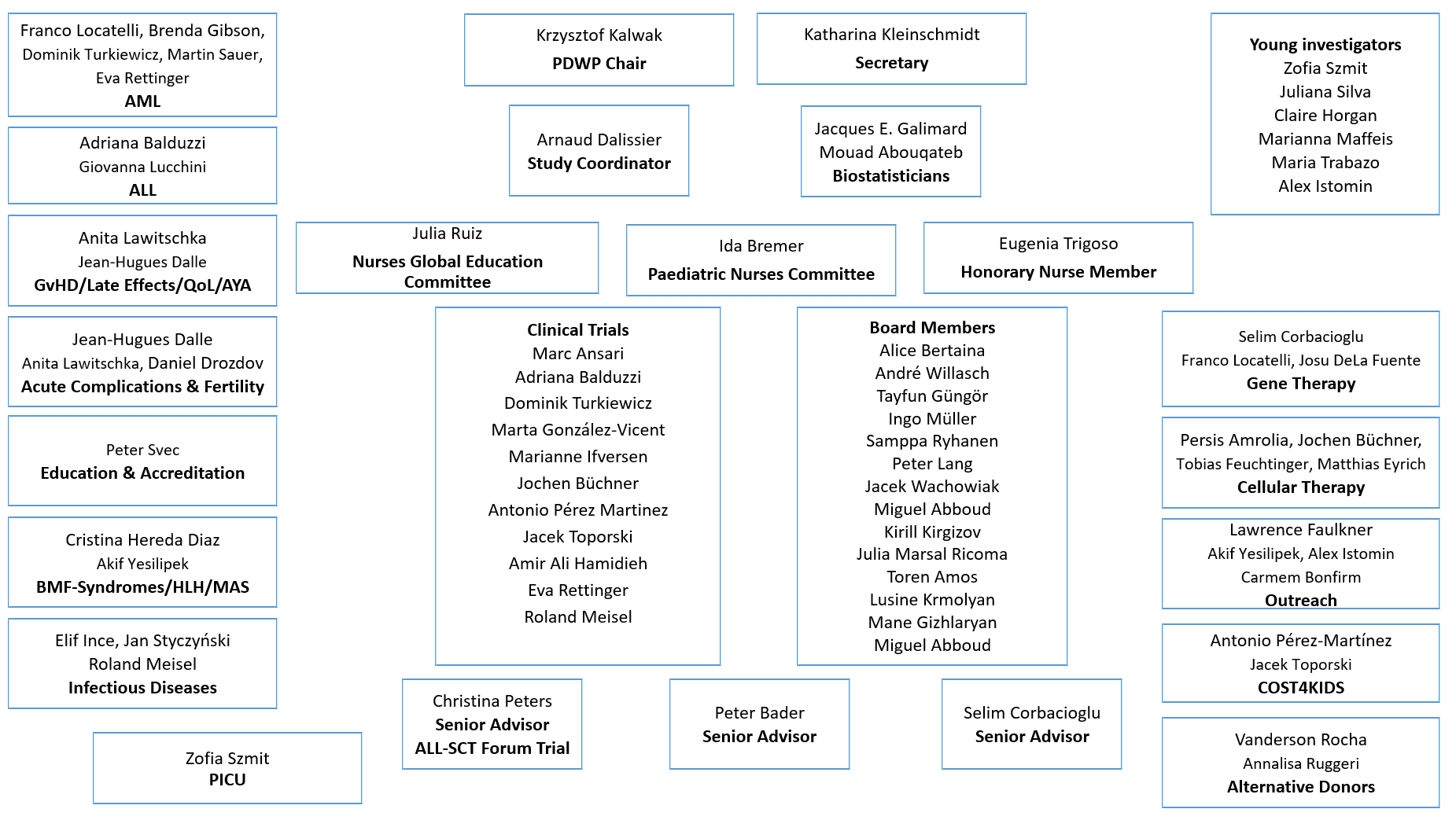
PDWP Team








Structure
PDWP Organigram